
November 8, 2010
Japan Agency for Marine-Earth Science and Technology
New Metabolic Pathway of Oceanic Benthic Archaea
- Recycling of Membrane Lipids -
Overview
Researchers from the Institute for Biosciences (Biogeos) and the Kochi Core Center of the Japan Agency for Marine-Earth Science and Technology (JAMSTEC), and the University of Bremen, Germany, have discovered evidence for a new metabolic pathway of the deep-sea benthic archaea(*1), which is believed to have evolved to maximize the scarce energy source in deep sea environment.
The study, led by Yoshinori Takano, and Naohiko Ohkouchi, Program Director of Biogeos, suggests that archaea take up organic molecules whose molecular weight of as large as 500 or more from the sediments and utilize them for synthesizing their own membranes. By “recycling” the organic matter readily available in the surrounding sediments, the archaea minimize energy expenditure for survival in deep sea sediment where energy sources are extremely limited.
The research paper was published in the November 7 online issue of the journal Nature Geoscience.
- Title:
- Sedimentary membrane lipids recycled by deep-sea benthic archaea
- Authors:
- Yoshinori Takano, Yoshito Chikaraishi, Nanako O. Ogawa, Hidetaka Nomaki, Yuki Morono, Fumio Inagaki, Hiroshi Kitazato, Kai-Uwe Hinrichs, and Naohiko Ohkouchi
Background
The deep ocean sediments are home to a variety of microbes that play an important role in the carbon cycle at the Earth’s surface. Archaea are among them, and inhabit a broad range of marine environments. Recent studies revealed that their distribution and biomass in seawater and bottom sediment are greater than previously thought, which has drawn the growing attention of scientists. Yet their ecology, even basic knowledge on microbial activity and metabolism, still remains elusive because of the difficulty of culturing them in the laboratory.
Novel techniques developed by the group successfully conducted an in-situ incubation of the deep-sea benthic archaea in Sagami Bay, Japan. The experiment results were analyzed on-shore using accurate biomarker analysis.
Method
Researchers placed 30 cm-long acrylic cylindrical chambers (called “cores”) on the seafloor at a water depth of 1,453m in Sagami Bay (Fig. 1), using JAMSTEC’s remotely operated vehicle “Hyper Dolphin.” Glucose labeled with 13C (stable carbon isotope) was carefully injected into the head space of the chamber through the syringes attached to it.
After 9 and 405 days of incubation, the core sediment was recovered and analyzed for ether lipids(*2), a component unique to archaeal membranes. Ether lipids are lipids in which a glycerol backbone is bonded to isoprenoid (*3) chains via an ether bond. Unlike other organisms (i.e., bacteria and eukaryotes) that have ester-linked membrane lipids(fig 2.), archaea use ether bonds to synthesize membrane lipids. After being isolated from the sediments, the ether lipid was chemically cleaved (broken apart) into glycerol(*4) and isoprenoid units. Each unit was analyzed to trace the uptake of the 13C-labelled glucose into the molecules.
Results and discussion
The results showed a pronounced assimilation of 13C in the glycerol unit, whereas the isoprenoid unit remained unlabelled. This indicates that the 13C-labelled glucose was used to synthesize the glycerol backbone, but was not for isoprenoid chains. Researchers suggest the presence of permeable membrane channels, through which archaea take up sedimentary isoprenoids resulting from the degradation of archaeal membranes into the cells and chemically bind them to glycerol to synthesize membrane lipids - a metabolic pathway involving “recycling” relic organic compounds.
Isoprenoid is a large molecule comprised of 40 carbon atoms, with a molecular weight of 562. The “recycling” mechanism of such a large molecules by archaea has been little known. This study provides evidence for the membrane channels through which large molecules can be incorporated (fig. 3).
The study group is now working to determine the biological activity of archaea (e.g. growth and reproduction rates) by measuring the 13C uptake rate into the isoprenoids. Given the results of their incubation experiment, the archaeal bioactivity is considered to be much greater than previously estimated, signaling their even more important role in the marine benthic carbon cycle.
Future perspective
The group will continue their study to better understand the entire global carbon cycle. The results are expected to make a broad range of contribution to society, including environmental restoration using microbial bioaugmentation as well as industrial use of compound-specific isotope analysis.
The scope of perspectives:
(1) Understanding of the global carbon cycle
Oceans cover around 70% of the Earth’s surface. Archaea live in the ocean, terrestrial water bodies and soil. Understanding the mechanism of archaeal membrane channels permeable to large isoprenoids and accurately evaluating the biological activity of the deep-sea benthic archaea are expected to unveil the role of archaea in the global environment and thus the global carbon cycle, which is crucially related to global environmental change. By enhancing the reliability of measuring the biomass of marine benthic archaea and of estimates in archaeal bioactivity in all oceans, the group will quantitatively simulate the archaeal contribution to the carbon cycle.
(2) Environmental restoration using microbes
Ether-bonds are chemically strong and stable to alkaline and acidic hydrolysis. Some ether compounds found in anthropogenic environmental pollutants (e.g. dioxin and nonylphenol) are harmful for life. The isotope analysis conducted in the study implies a large population of benthic microbes capable of cleaving ether-bonds in compounds. If we can identify such microbes, isolate and grow them in the laboratory, it will be possible to use them for the remediation of environmental pollution.
(3) Innovation by compound-specific isotope analysis
One of the key techniques employed in this study is the compound-specific analysis, a novel method capable of tracing and characterizing isotopic compositions of organic compounds. The high-precision isotopic analysis will not only enhance knowledge in microbial ecology and biogeoscience, but also benefit a wide range of industrial applications such as environmental monitoring, pharmaceutical development, and sectors requiring a strict quality control or analysis of material dynamics. These are expected to contribute both to green innovation (innovation in environmental technologies) and “life innovation” (innovation in medical and nursing care technologies).
- *1.
- Achaea
All life forms on Earth are divided into three domains: archaea, bacteria, and eukarya. Most microbes belong to archaea or bacteria; these two microbes can be differentiated by the polar lipid structure in their cell membranes. - *2.
- Ether lipids
Ether lipids are the main membrane constituents of archaea. An ether lipid molecule consists of a glycerol backbone and isoprenoid side chains attached to it via an ether linkage. The ether linkage, composed of one oxygen and two carbon atoms, is unique to archaea. Bacterial side chains are bound using an ester linkage. - *3.
- Isoprenoids refer to a group of organic compounds synthesized in the cell. The isoprenoid of interest in this study is built from 8 isoprene molecules, containing 40 carbon atoms and with a mass number of 562. An isoprene molecule is built by 5 atoms and 8 hydrogen atoms.
- *4.
- Glycerol
Glycerol is a naturally occurring alcohol with a mass number of 92. It contains 3 carbon atoms and constitutes lipids.
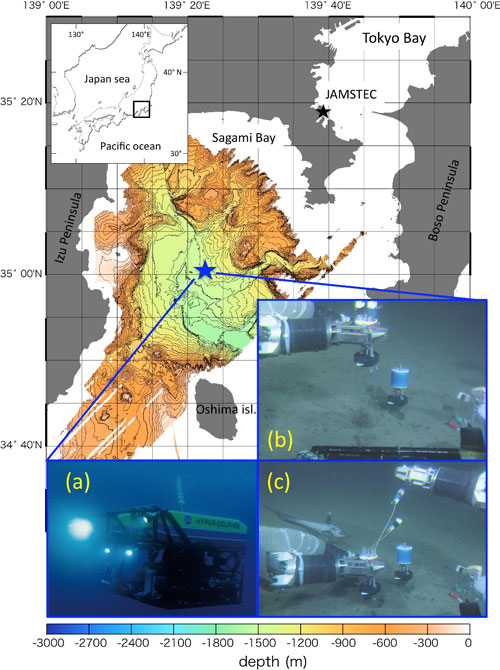
Figure 1: Study site: (a) Hyper Dolphin; (b) and (c) Installation of the incubation cores on the seafloor in Sagami Bay (water depth:1453 m), using the ROV Hyper Dolphin. Injection syringes are attached atop the cores.
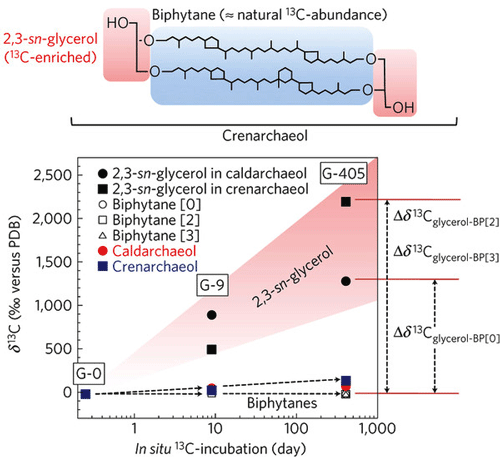
Figure 2: Carbon isotopic compositions of δ13Ccaldarchaeol, δ13Ccrenarchaeol, δ13Cbiphytane and δ13C2,3-sn-glycerol during the course of the experiment. 13C levels in glycerol units increase in time, showing the consumption of 13C-glucose in glycerol synthesis. In contrast, 13C levels in isoprenoids remained unchanged, indicating the absence of 13C-glucose. 13C levels in caldarchaeol showed a similar trend.
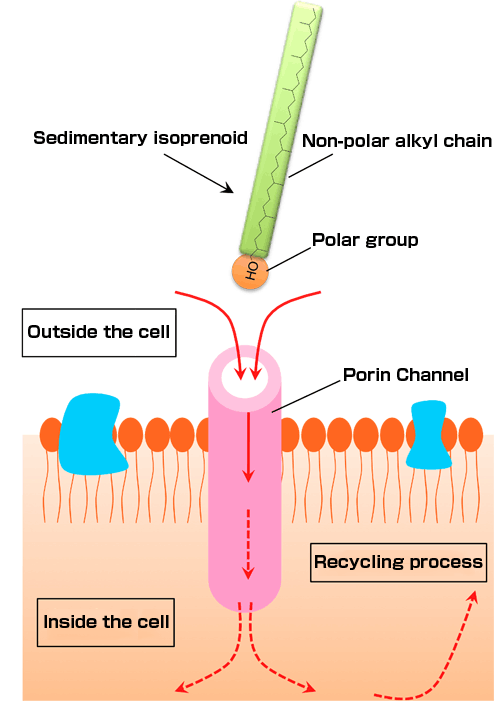
Figure 3: Hypothetic process to incorporate isoprenoids through membrane channels
Isoprenoids have a hydrophilic polar group (hydroxyl group) at the end of the chain, by which they can attach to a hydrophilic protein at membrane porin channels and pass through the opening. Inside the cell, they are restructured to other organic compounds and used for survival.
Contacts:
- Japan Agency for Marine-Earth Science and Technology
(For the study) - Naohiko Ohkouchi, Program Director
Yoshinori Takano, Scientist
Earth and Life History Research Program
Institute of Biogeosciences (Biogeos)
- (For publication)
-
Toru Nakamura, e-mail: press@jamstec.go.jp
Manager, Planning Department Press Office