Press Releases


JAMSTEC
The University of Tokyo
Unique Mechanism of Iron Sulfide Production in Scaly-foot Snail Revealed
1. Key points
- ◆
- The process by which iron sulfide is produced and incorporated into the unique scales of the scaly-foot snail, endemic to deep-sea hydrothermal vents, was revealed using cutting-edge microscopy.
- ◆
- The mechanism by which the scaly-foot snail mineralizes iron sulfide differs from other known biomineralization processes in that only half of the chemical species are contributed by the animal itself: the snail secretes sulfur into its scales, where it then slowly reacts with iron ions from the vent fluid diffusing in from the surrounding environment.
- ◆
- This discovery implies a link between iron sulfide mineralization and symbiosis in the scaly-foot snail, and indicates that the discarding of metabolites from symbionts may be a more widespread key survival strategy in chemosynthetic ecosystems.
2. Overview
The research group led by Satoshi Okada of the Research Center for Bioscience and Nanoscience of the Research Institute for Marine Resources Utilization at the Japan Agency for Marine-Earth Science and Technology (JAMSTEC) has, in collaboration with scientists at the University of Tokyo, revealed the mechanism by which the scaly-foot snail incorporates iron sulfide nanocrystals into its scales. The scaly-foot snail is a marine gastropod, endemic to deep-sea hydrothermal vents in the Indian Ocean. It is noted for having dense scales on the surface of the soft, exposed part of its body (Figure 1) and sulfur-oxidizing endosymbiont bacteria on which it relies for energy. Previous research has shown the presence of iron sulfide nanoparticles inside the scales. However, it has not yet been clarified whether these particles are produced through biological processes.
The research group utilized elemental microanalysis as well as cutting-edge electron microscopic techniques to identify that the iron sulfide minerals found within the scales of the scaly-foot snail are indeed of biological origin (Figures 2 & 3). In particular, they were the first to discover time that the snail secretes sulfur into its scales, where it then reacts with iron that diffuses in from the surrounding water to produce iron sulfide (Figure 4). Two different sources of minerals are involved in the mineralization mechanism, unlike other animals in which materials for mineralization are sourced from a single mechanism. As the sulfur likely originates from metabolites of the snail’s endosymbionts which the snail discards through its scales, convergent evolution is likely to have led to the development of similar mechanisms in other deep-sea chemosymbiotic animals.
Iron sulfides produced by the scaly-foot snail include pyrite (FeS2), which is a semiconductor, and greigite (Fe3S4), which has magnetic properties. Currently, the industrial production of these sulfide minerals requires high temperatures, but the scaly-foot snail is able to produce these minerals at much lower environmental temperatures (i.e., 10–20°C), close to room temperature. It is likely that the biomimicry of the scaly-foot snail’s mineralization mechanism will enable the more efficient production of iron compounds, and also further the development of a vast array of functional inorganic materials.
The results of this study were published in the Proceedings of the National Academy of Sciences of the United States of America on September 10th, 2019 (JST). This research was supported by the Japan Society for the promotion of Science (JSPS), as Grants-in-Aid for Scientific Research (Grant Nos.: JP16H07490, JP22540499, and JP26287133).
- Research Center for Bioscience and Nanoscience, Research Institute for Marine Resources Utilization, JAMSTEC
- X-STAR, JAMSTEC
- Department of Food and Nutrition, Higashi-Chikushi Junior College
- Department of Earth and Planetary Science, Graduate School of Science, The University of Tokyo
- Department of Chemical Oceanography, Atmosphere and Ocean Research Institute, The University of Tokyo
- Institute of Surface-Earth System Science, Tianjin University
- Department for Continental Shelf, Maritime Zones Administration & Exploration, Ministry of Defence and Rodrigues, Port-Louis, Mauritius

Figure 1 Scaly-foot snails collected from the Kairei vent field infused with iron sulfide (left) and from the Solitaire vent field without any iron sulfide mineralization (right).
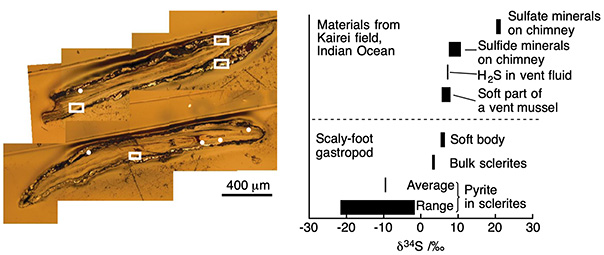
Figure 2 Elemental microanalysis of cross-sections of scales of a scaly-foot snail from the Kairei vent field. The white dots and squares (left) were irradiated with an ion beam to measure the ratio of sulfur isotopes present in the scale. These ratios were then compared with those of the vent fluid and the muscles of the snail and other animals taken from the same environment (right). The ratio of the heavy 34S isotope was the lowest within the interior of the scales.
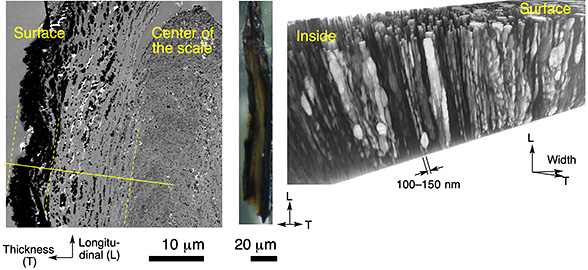
Figure 3 Cross-sectional image of scale (left) and 3D reconstruction of the distribution of the included iron sulfide minerals (right). The dark regions in the cross-section and the bright regions in the 3D image represent iron sulfide minerals. The iron sulfide particles decrease in size from the surface toward the interior of the scale, and are aligned in a channel-like manner along the long axis of the scale.
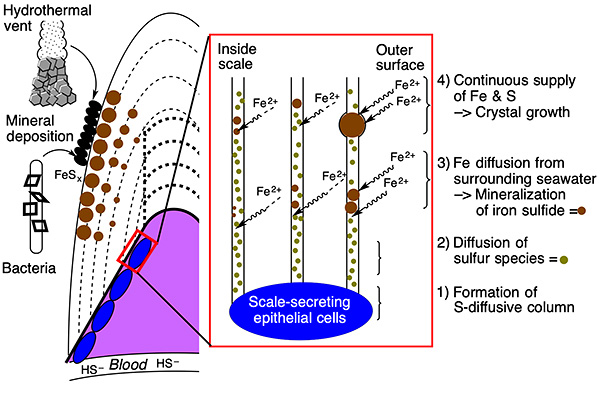
Figure 4 Schematic drawing summarizing the mechanisms by which the scaly-foot snail produces the iron sulfide minerals observed on the interior and exterior of its scales.
Contacts
- (For this study)
- Satoshi Okada, Scientist, Research Center for Bioscience and Nanoscience, Research Institute for Marine Resources Utilization, JAMSTEC
- Yohei Suzuki, Associate Professor, Department of Earth and Planetary Science, Graduate School of Science, The University of Tokyo
- Yuji Sano, Professor, Atmosphere and Ocean Research Institute, The University of Tokyo
- (For press release)
- Public Relations Section, Marine Science and Technology Strategy Department, JAMSTEC
- Public Relations, School of Science, The University of Tokyo
- Public Relations Office, Atmosphere and Ocean Research Institute, The University of Tokyo