2. Warming and atmospheric composition change interaction model developmentResults Page | Top Page |
||||||||||||||||||||||||||||||||||||||||||||
|
2?1 Warming and atmospheric composition change interaction (atmospheric chemistry) The organization in charge: Earth frontier research system
a. SummaryIn warming and atmospheric composition change interaction sub model, it is the present subject to enable on-line calculation of aerosol and chemistry using all the ball chemistry models CHASER and the aerosol model SPRINTARS which set it as the main purposes to express and predict an interaction with warming of atmospheric chemistry process (ozone distribution etc.) or aerosol, and the ocean and terrestrial vegetation change, and used CCSR/NIES AGCM as the foundation. In the current fiscal year, the CHASER model was accelerated bearing in mind the long-term experiment at the time of including this sub model in an integrated model, and it evaluated on the Earth Simulator about the execution performance. Large calculation cost reduction was realized by this high speed work about the chemical process. Furthermore, the future prediction experiment of the troposhere chemistry place which took warming into consideration as preceding paragraph story-research for warming and atmospheric chemistry interaction prediction was conducted. Especially this fiscal year analyzed preponderantly change of the stratosphere / substance exchange between the troposhere at the time of warming about this prediction experiment, and obtained the prediction result of the ozone inflow from the stratosphere to the troposhere increasing by change of the atmospheric circulation place by warming. Moreover, combination of CHASER and a SPRINTARS car model was started in the second half of this fiscal year. About verification of a transport process currently performed from the last fiscal year, especially this fiscal year calculated age distribution of air as evaluation of transportation in the stratosphere, and transportation in the stratosphere, and compared with the observed value in the lower stratosphere. Although the value near an observation point estimate was acquired in the equatorial region in this calculation, it turned out in inside and high latitude that it is in the tendency which underestimates the average age of air. b. Research purposeThe ozone in the troposhere is powerful greenhouse-effect gas, aerosol is also strong to sunlight reflection and absorption, and generation of clouds, and involves [ both ], and influences climate. Moreover, ozone participates in generation of a hydroxylation radical (OH) directly in the troposhere, and influences the chemical life of other greenhouse-effect gas, such as methane and halocarbons (CFCs), (Fig. 16). Furthermore, since troposhere ozone and sulfuric-acid aerosol hold substantially the key of atmospheric environment change represented by acid rain etc., it is important how these are changed by future human activities (especially East Asia region). Moreover, since there are some which have the chemistry of the troposhere, such as secondary organici matter aerosol (SOA) produced in the oxidization process of sulfuric-acid aerosol or hydrocarbon, and strong relevance in an aerosol kind, also in case future aerosol distribution and the influence on the climate are considered, it is necessary to use the model combined with the chemical process. Furthermore, the place where the chemical process of the troposhere is influenced by the conditions (climate) of weather places, such as steam, temperature, and circulating space, is large (for example, Sudo et al., 2001, 2003). Therefore, the model which can calculate ozone aerosol distribution simultaneously in the framework of a climate model in order to aim at more advanced climate change and atmospheric environment change prediction, and can examine an interaction change process of climate and ozone aerosol needs development and to be developed. In the framework of the 2nd subject of this symbiosis project, the troposhere / stratosphere chemistry, and the climate model in which on-line calculation of aerosol is possible are built by using a CHASER-SPRINTARS car model as a foundation under such a background, and the interaction of chemistry and aerosol change, and a climate change is predicted and studied.
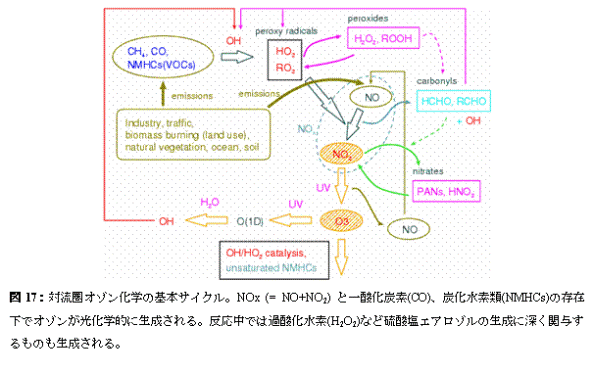
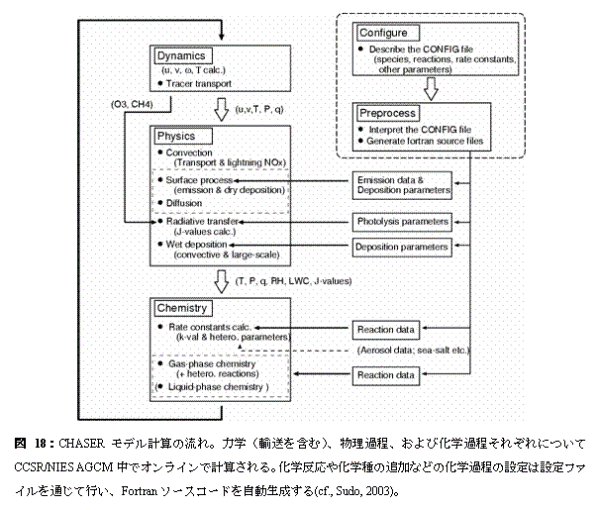
c. A research program, a method, a scheduleIn this subgroup research, model development and research centering on all ball chemistry and the climate joint model CHASER (Sudo et al., 2002a) are done. A CHASER model is the University of Tokyo climate system research center (CCSR), They are an earth frontier research system (FRSGC) and all the ball chemistry models currently developed jointly in National Institute for Environmental Studies (NIES), the inside of a CCSR/NIES climate model -- the photochemical reaction in the atmosphere, human work and natural origin outgassing (emission) process, and earth surface -- detailed chemical processes, such as build-up (deposition) process by - precipitation, are taken into consideration on-line (Table 2: : which is taking into consideration the chemical reaction system centering on troposhere ozone as a chemical reaction in the present setup figure 17). Distribution of the ozone (O3) calculated by the CHASER model, or precursor gases (nitrogen oxide NOx, carbon monoxide CO, hydrocarbon VOCs, etc.) and important related gas shows quantitive very good coincidence as the various observation data using a satellite or an airplane, and, also globally, goes a tip as simulation capability of troposhere ozone chemistry (Sudo et al., 2002b). Moreover, in a CHASER model, it is Fig. 18 That which generates a model code (Fortran) automatically by the Puri processor through a configuration file about chemical species or a chemical reaction system, and change and an addition of chemical species and a reaction are easy. This research program aims at the ozone chemical process of the troposhere and the stratosphere, and construction of the chemistry and the aerosol joint climate model in which the simultaneous simulation of various aerosol is possible by using a CHASER model as a foundation.
Although all the ball chemistry models CHASER mainly target troposhere chemistry in a present condition setup, compared with standard AGCM, its calculation cost is very large including various chemical reactions. Then, in advance of the introduction of stratosphere chemistry and aerosol calculation including the ozone hole to CHASER, CHASER is accelerated (especially chemical process) and the execution performance on an Earth Simulator is evaluated. Moreover, after cooperating with a "climate physics core model improvement" subgroup, about the perpendicular resolution as an integrated model etc., he examines based on this evaluation and it is decided that it will be it (FY 2003). Furthermore, the work which combines the aerosol model SPRINTARS (Takemura et al., 2002) with the accelerated CHASER model is started. About the inside of an aerosol kind, and sulfuric-acid aerosol, the generation process is hydrogen peroxide (H2O2), a hydroxylation radical (OH), ozone distribution, etc.
d. The research program in FY 2003
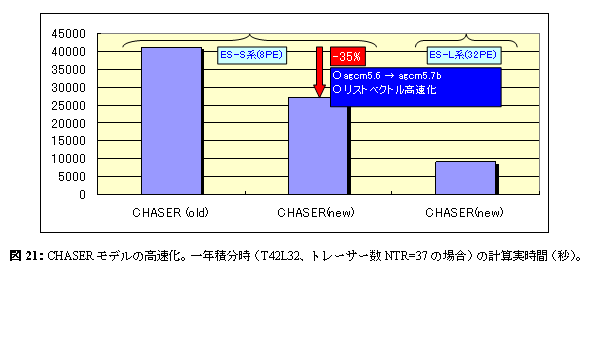
The troposhere chemistry model CHASER developed in the University of Tokyo climate center is extended, a stratosphere chemical reaction is incorporated, and high resolution time slice simulation is performed. However, CHASER is an implication about very many variables. e. Reports in FY 2003e.1. Improvement in the speed of the chemical-bond climate model CHASERIn all ball chemistry models, it is a pending question element at the time of the calculation cost of a chemical process (especially chemical reaction) being very large, and conducting a long-term experiment as compared with a basic climate model. moreover, since increasing further is expected, the calculation cost at the time of also introducing aerosol and stratosphere chemistry (the present condition -- troposhere chemistry) into CHASER as an integrated model needs to accelerate calculation of a chemical process as much as possible. While shifting CHASER which was CCSR/NIES agcm5.6 base to the agcm5.7b base in the current fiscal year, chemistry related process was accelerated. It succeeded in reducing the whole computation time about 35% by having mainly introduced the list vector(list-vector)-ized technique into the chemical process, as shown in Fig. 20 (when an Earth Simulator or a NEC-SX calculus-of-vectors machine is used). About list vectorization, it carried out for the conditional execution block (conditional vector operation) of if sentence etc., and reduction of efficient calculation cost was realized in various chemical processes, such as generation of the liquid phase chemical reaction relevant to clouds, wet deposition (wet deposition), and NOx from thunder.
Moreover, although simplifying a chemical reaction system should also take into consideration in the aspect of the calculation cost reduction of a chemical process, it is checking that about 20% of improvement in the speed is still more possible by reduction of the middle generation chemical substances (superoxide etc.) and the number of tracers which are taken into consideration by the present CHASER.
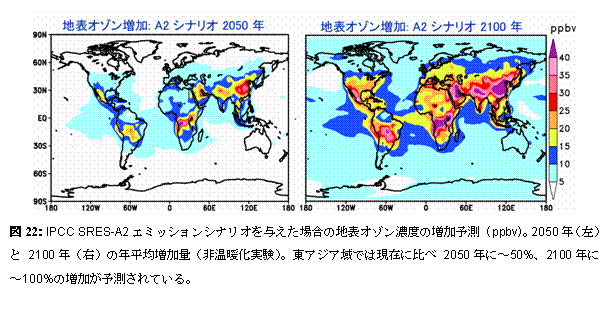
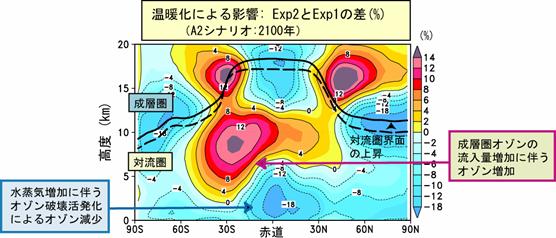
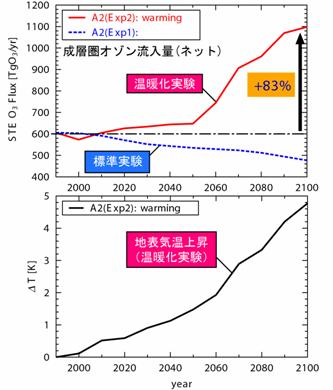
The further high speed work of introducing also into a chemical process (especially vapor phase chemical reaction) the MPMD (Multi Process Multi Data) technique adopted as AGCM physics process etc. from now on is planned. e.2. Chemistry and a warming future prediction experiment : influence on the troposhere chemistry by warmingIn order to acquire the prospect of warming and an atmospheric composition change interaction, future warming is experimenting using CHASER about the influence (feedback) which it has on a chemical process (especially ozone distribution). Here, the future prediction experiment of contaminants, such as troposhere ozone and sulphate aerosol, currently conducted by the CHASER model based on each scenario proposed from IPCC is reported. In this research, the experiment (Exp1) of only discharge (emission) change of precursor gases ozone, such as nitrogen oxide NOx and carbon monoxide CO, and two kinds of experiments of the experiment (Exp2) which also took the climate change into consideration in addition to emission change were performed. In these experiments, a future emission change and a climate change gave the sea surface temperature (SSTs) and sea ice (Sea-ice) distribution which were predicted by the CCSR/NIES air sea joint model while making greenhouse-effect gas concentration, such as CO2, increase in a model about the climate change of Exp2 according to both IPCC SRES-A2 scenarios. Especially in the experiment (Exp1) in consideration of change of only emission change of precursor gases ozone, photochemistry ozone generation increased in the East Asia region where the increase in emission is large, and the remarkable increase in surface-of-the-earth ozone was calculated (Fig. 22). Moreover, since ozone generation was strengthened by active perpendicular transportation of precursor gases from surface of the earth also in the up troposhere of the East Asia domain, it rode on westerlies and it turned out that the influence in all ball scales is very large. In this experiment (Exp1), the increase in an almost linear troposhere ozone total amount was calculated from 1990 to 2100, 23% was predicted in 2050 and about 40% of increase was predicted in 2100. However, when the influence of future global warming also took the change process of the troposhere ozone by such increase in emission into consideration, it turned out that a situation changes. It is shown what kind of difference comes out of Fig. 23 to east-and-west average ozone distribution by the case (Exp1) where it is not considered as the case (Exp2) where warming is also taken into consideration in the prediction experiment in 2100. If warming is taken into consideration, in a troposhere lower layer, since ozone becomes destroyed [ tend ] by the increase in steam, ozone will decrease, and the ozone reduction accompanying a tropopause rise will be seen in the up troposhere of high latitude. On the other hand, although the increase in ozone was calculated by warming in the concave latitude up troposhere, it was checked that this is the result of strengthening the meridional circulation (Buruwa Dobson circulation and Hadley circulation) of the stratosphere and the troposhere with advance of warming, and the ozone inflow from the stratosphere increasing greatly (Fig. 24). Moreover, although prediction of methane or sulphate aerosol was also performed in this experiment, it checked changing being greatly dependent on change of the ozone chemistry places (OH radicalness, H2O2 concentration place, etc.) by emission change, and a change according to warming further through a chemical reaction also about these future distribution. Thus, it was suggested to future prediction of the troposhere ozone chemistry place containing methane or aerosol that it is necessary to take not only emission change but the influence by warming into consideration. It experiments about other scenarios of IPCC SRES from now on, and the sensitivity by emission change or a climate change is considered in more detail.
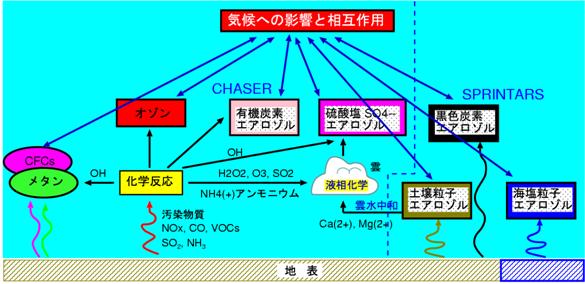
Although the simultaneous on-line simulation of chemistry and aerosol was planned by the framework of a K2 integrated model, this fiscal year started the work which introduces into the chemistry model CHASER the aerosol process which used SPRINTARS (abbreviated version) as the foundation. As specifically shown in Fig. 25, combination of the simulation of ozone chemistry and various aerosol simulations is performed. Although it applies to a SPRINTARS model among aerosol kinds about calculation of black carbon aerosol (Black Carbon:BC), a sea salt particle (Sea-salt), and a soil particle (Soil-dust), about a sulphate (Sulfate) with strong relation and secondary organic carbon aerosol (SOA) with chemistry, it joins together by linking with the chemical reaction process of CHASER. Under the present circumstances, since the value of cloud underwater pH is important about the sulphate generation process by liquid phase oxidization of shift out2, in consideration of the neutralization process by basic substances, such as a soil particle (dust: calcium2+, Mg2+) and ammonium (NH4+), it is considered as a more realistic simulation. Moreover, introduction of an aerosol thermal equilibrium model is performed for the simulation of ammonia (NH3) ammonium (NH4+). Furthermore, it enabled it to treat the generation from the oxidation reaction by the ozone of the terpenes of the vegetable origin by the chemical reaction of CHASER about secondary organic carbon aerosol in the present condition. Although the great portion of above chemistry and aerosol joint work are completed at the present stage, a program check and a test experiment are conducted from now on, and the check work of the validity (itinerant Buddhist pH etc.) of an optical parameter or a sulphate simulation in connection with absorption and dispersion of aerosol is done especially. Moreover, in advance of a transplant to the integrated model (KISSME) of this chemistry and aerosol joint model, it evaluates about the execution performance on an Earth Simulator, and the high speed work of MPMD etc. is considered.
e.4. Examination of a stratosphere chemical process It is very important for halogenated compounds, such as chlorine and bromine, in a stratosphere chemical process to take the reaction system of these compounds into consideration in order to play a role important for the ozone destruction in the stratosphere according to the catalytic cycle action. The reaction system used by stratosphere photochemistry models (Takigawa et al., 1999 and 2001, Nagashima et al., 2002, Akiyoshi et al., 2002, etc.) was added to CHASER towards mounting of the stratosphere chemical process after the following fiscal year, and calculation speed using a box model was presumed (Table 3).
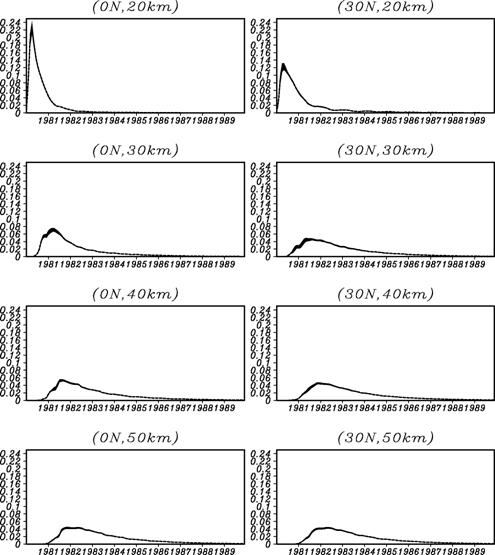
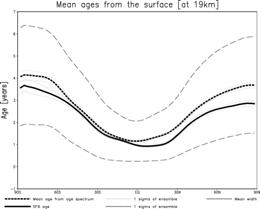
Since it is necessary to newly take into consideration the reaction of long-life life gas, such as methane and a dinitrogen oxide, etc. in addition to a chlorine system compound, Table 3 shows [ at present ] that may be become heavy more than twice, when even a chlorine system compound is added. Moreover, even if it puts in even a bromine system compound from the result of this box model, it does not necessarily become so heavy. In case it actually mounts in an integrated model, I want to examine introducing the reaction system which includes even a bromine system compound first from these results. Moreover, since the halogenated compound generated as a result of the heterogeneous reaction on the polar stratospheric cloud (Polar Stratospheric Cloud:PSC) surface is Cl2 which is easy to carry out maceration by a photochemical reaction, for evaluation of the amount of stratosphere ozone destructions by an ozone hole, it is very important for it to reproduce correctly the decay time of a polar vortex, the radiant flux in the circumference part of polar night, etc. For this reason, into our group, improvement of a gravity wave resistance scheme, introduction of the surface-of-a-sphere effect in a radiation scheme, etc. are due to be performed, cooperating with a dynamics core subgroup etc. closely. Among these, about the surface-of-a-sphere effect, it is already introductory ending at the stratosphere photochemistry model of a former version, and it is due to introduce into the stratosphere version CHASER after aiming at further improvement in calculation efficiency during [cf., Kurokawa et al., 2002, Nagashima et al., 2003], and FY 2004. A numerical simulation in a high resolution model is just going to be done in the way of a dynamics core subgroup energetically also about the gravity wave resistance scheme, and after carrying out a parameter rise appropriately for inside resolution models based on these results from now on, it introduces into the stratosphere version CHASER. e.5. Verification of a transport processIt is important to evaluate the transport process in CCSR/NIES AGCM quantitatively, also when raising the reliability over the result of material-recycling research, and it continued at the last fiscal year, and conveying space using a passive tracer was verified. Its attention is paid to the stratosphere especially in this research, and they are Kida [1983] and Hall and Plum. It was used by [1994]. Age presumption of stratosphere air mass using Age Spectrum was performed, and comparison with the actual age presumed from the result of aircraft observations, such as carbon dioxide in the lower stratosphere, was performed. (Experiment) The passive tracer n introduced in order to calculate Age Spectrum assumed and gave Dirac's delta-function within the equatorial region surface boundary layer. That is, the sauce of a steady value is given for one month of the beginning, 0 is given about the period after it, and this domain becomes a sink. Moreover, advanced P, time Tracer in t Mixing ratio of n,
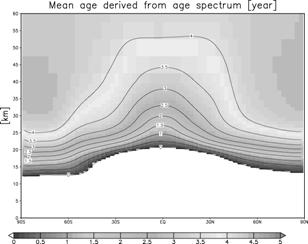
(Result) It can set to the equator, the stratosphere of north latitude 30 degrees, and a mesosphere figure 26. Age Spectrum is shown. The time of a peak is overdue as an altitude becomes high, and signs that distribution is becoming blunt are seen. Moreover, signs that the way of inside latitude is behind the equator in the peak hour term are also seen. Although line width shows 1sigma of an ensemble experiment collectively, although the influence near a peak value is seen slightly, it is at most about 3% as a grade also in the inside latitude lower stratosphere, and it can be considered that the influence of the peak position and peak intensity on Age Spectrum by the difference in a dynamics place is a small thing. Mean residence time gamma in each place of the stratosphere The primary moment of Age Spectrum is used,
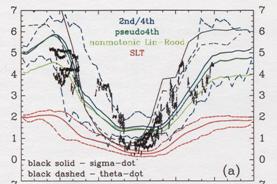
Next, 4 from which CO2 aircraft observation and an advection current scheme differ the north-south concentration gradient in the lower stratosphere - a kind GFDL SKIHI The thing in comparison with the point estimate [Eluszkiewicz et al., 2000] by the model and a trajectory model is shown in Fig. 28. In order to compare with an observation result etc., these results differ in Fig. 27 and are expressed with the age on the basis of surface of the earth. The result of this experiment is well in agreement in the value and equatorial region of a point estimate (Fig. 27 top, sunspot) from observation. On the other hand, this result has a small concentration gradient between the latitude in equatorial - as compared with observation, and it turns out that the model has underestimated the so-called sub--tropicalbarrier. GFDL SKIHI on Fig. 27 This is the general tendency of a three dimension model, and the result of having used the almost same scheme (nonmonotonic Lin--Rood) especially is conformable so that it may understand, even if it sees the result of a model. Generally this model showed the low-temperature tendency in the equatorial region lower stratosphere as compared with re-analysis data etc., and, as a result, has underestimated the lead direct electric current in the equatorial region lower stratosphere as compared with observation. These may have influenced the vertical and meridional transport in an equatorial region. (Conclusion) age presumption of stratosphere air mass using a CCSR/NIES general circulation model -- a passive tracer -- and -- It carried out using Age Spectrum. Consequently, it turned out that the time of the result depended on each experiment of an ensemble experiment is small, that the north-south concentration gradient in the lower stratosphere is small as compared with observation, etc. Although experimented this time considering the generation source of a passive tracer as inside of 1 - the equatorial region surface boundary layer of the moon, I want to conduct the experiment which put the generation source on other time and latitude belts from now on, and to evaluate what kind of influence it has on Age Spectrum etc. Moreover, horizontal resolution was changed, the same experiment was conducted and it considers investigating influence on sub--tropical barrier. In order to evaluate the influence of the low-temperature bias in the equatorial region lower stratosphere collectively, the experiment using the radiation scheme which removed this is also planned.
f. ConsiderationThis fiscal year did the high speed work of the chemistry model CHASER in advance of aerosol introduction first. Although it succeeded in reducing the whole computation time about 35% by introducing the technique of a list vector into a chemical process in this improvement in the speed, integration will take 2.5 hours (real time) by an Earth Simulator L system (32PE) by the present setup (T42L32) for one year, and concern will still be left behind to the calculation cost as an integrated model at the time of adding aerosol and stratosphere chemistry to CHASER. The present condition is considering simplification of the chemical reaction system of the troposhere using a 0-dimensional point model especially in order to reduce calculation concerning chemical reaction calculation, but it is also the further subject to introduce the parallel techniques, such as MPMD, into chemical reaction calculation. This fiscal year analyzed in detail the influence future warming affects ozone distribution about the preceding paragraph story-experiment of the atmospheric chemistry change and the warming interaction which used CHASER. A possibility that the ozone distribution of the troposhere and the lower stratosphere would receive change remarkably by change of the large-scale atmospheric circulation (stratosphere / troposhere circulation) accompanying warming was shown by especially the analysis of this fiscal year. Moreover, it checked that generation of sulphate aerosol was also greatly influenced by warming. Since ozone and sulphate aerosol in the troposhere are also main contaminants while they are a climate influence substance, not only the influence on climate but its influence on environment is large. Therefore, playing an important role in respect of two, future "climate change prediction" and "environmental change prediction", as the chemistry and an aerosol joint climate model which is building into this group is expected. It is planning conducting the prediction experiment which clarified influence of the climate on troposhere ozone and aerosol as future research directivity, and followed all the scenarios of IPCC. Although this fiscal year also did the work which combines the aerosol simulation based on abbreviated version SPRINTARS with CHASER, evaluation and improvement in the speed of this model are the further subject. Moreover, also in case it finally experiments by an integrated model, it assumes considering a future change process from an aspect of the interaction of chemistry, aerosol (especially a sulphate, organic carbon aerosol), and climate. Although the validity of the troposhere, the substance exchange between the stratosphere, and transportation in the stratosphere was estimated by the verification experiment of the transport process using age distribution of air, it is on GCM perpendicular coordinates. hybrid Investigation is required succeedingly also about the case where a coordinate system and a new radiation code are used. The following fiscal year (FY 2004) is planning the addition of a transplant to the integrated model (KISSME) of chemistry and an aerosol joint model (CHASER-SPRINTARS), and the stratosphere chemical reaction to CHASER as K2 integrated model development. g. Bibliography
Akiyoshi H., T. Sugata. S. Sugita, H. Nakajima, H. Hayashi, J. Kurokawa, M. Takahashi, Lower-N2O air masses after the breakdown of the arctic polar vortex in 1997 simulated by the CCSR/NIES nudging CTM, J. Meteorol. Soc. Jpn, 80, 451-463, 2002.
Eluszkiewicz, J., R.S. Hemler, J.D. Mahlman, L. Bruhwiler, and L.L. Takcs, Sensitivity of Age-of-Air Calculations to the Choice of Advection Scheme, J. Atmos. Sci., 57, 3,185-3,201, 2000.
Hall, T.M., and R.A. Plumb, Age as a diagnostic of stratospheric transport, J. Geophys. Res., 99, 1,059-1070, 1994.
Kida, H., General Circulation of Air Parcels and Transport Characteristics Derived from a Hemispheric GCM, Part2. Very Long-Term Motions of Air Parcels in the Troposphere and Stratosphere, J. of Meteor. Soc. Jpn., 61, 510-522, 1983.
Lin, S.-J., and R.B. Rood, 1996, Multidimensional flux-form semi-Lagrangian transport schemes, Mon. Wea. Rev., 124, 2,046-2,070, 1996.
Maiss, M., and I. Levin, Global increase of SF6 observed in the atmosphere, Geophys. Res. Lett., 21, 569-572, 1994.
Nagashima, T., M. Takahashi, M. Takigawa, and H. Akiyoshi, Future development of the ozone layer calculated by a general circulation model with fully interactive chemistry, Geophys. Res. Lett., 29, 10.1029/2001GL014926, 2002.
Nagashima, T., M. Takahashi, H. Akiyoshi, and M. Takigawa, The effects of non-orographic GWD scheme and radiation from large SZA on the Antarctic ozone hole, Process-oriented validation of coupled chemistry-climate models, 2003.
Sudo, K. and M. Takahashi, Simulation of tropospheric ozone changes during 1997-1998 El Nino: Meteorological impcat on tropospheric photochemistry, Geophys. Res. Letters., 28, 4091-4094, 2001.
Sudo, K., M. Takahashi, J. Kurokawa, and H. Akimoto, CHASER: A global chemical model of the troposphere 1. Model description, J. Geophys. Res., 107, 10.1029/2001JD001113, 2002a.
Sudo, K., M. Takahashi, and H. Akimoto, CHASER: A global chemical model of the troposphere 2. Model results and evaluation, J. Geophys. Res., 107, 10.1029/2001/JD001114, 2002b.
Sudo, K., Changing process of global tropospheric ozone distribution and related chemistry: a study with a coupled chemistry GCM, Doctoral Dissertation, Dept. of Earth and Planetary Science, Graduate School of Science, Univ. of Tokyo, 187pp, 2003.
Sudo, K., M.Takahashi, and H.Akimoto, "Future changes in stratosphere-troposphere exchange and their impacts on future tropospheric ozone simulations", Geophysical Research Letters., 30, 24, 2256, doi:10.1029/2003GL018526, 2003.
Takemura, T., T. Nakajima, O. Dubovik, B.N. Holben, and S. Kinne, Single-Scattering albedo and radiative forcing of various aerosol species with a global three-dimensional model, Journal of Climate, 15, 4, 333-352, 2002.
Takigawa, M., M. Takahashi, and H. Akiyoshi, Simulation of ozone and other chemical species using a center for climate system research/national institute for environmental studies atmospheric GCM with coupled stratospheric chemistry, J. Geophys. Res., 104, 14,003-14,018, 1999.
Takigawa, M., M. Takahashi, and H. Akiyoshi, Simulation of stratospheric sulfuric acid aerosol using a Center for Climate System Research / National Institute for Environmental Studies atmospheric GCM with coupled stratospheric chemistry: Part I, nonvolcanic simulation, J. Geophys. Res., doi: 10.1029/2001JD001007, 2002.
introduction of the air surface-of-a-sphere effect to Junichi Kurokawa, Eiji Akiyoshi, Tatsuya Nagashima, Hideaki Nakane, Hirohiko Masunaga, Teruyuki Nakajima, Masaaki Takahashi, a CCSR/NIES stratosphere Nashingu chemistry transportation model, and a photochemistry joint model, the collection of the Meteorological Society of Japan spring convention lecture drafts in FY 2002 , and p -- 346 and 2002. h. The announcement of a result<Paper announcement>
Sudo, K., Takahashi, M., and Akimoto, H., "Future changes in stratosphere-troposphere exchange and their impacts on future tropospheric ozone simulations", Geophysical Research Letters., 30, 24, 2256, doi:10.1029/2003GL018526, 2003. <Oral announcement>
Nagashima, T., M. Takahashi, H. Akiyoshi, and M. Takigawa, The effects of non-orographic GWD scheme and radiation from large SZA on the Antarctic ozone hole, Process-oriented validation of coupled chemistry-climate models, 2003.
Sudo, K., Takahashi, M., and Akimoto, H., "Future changes in stratosphere-troposphere exchange and their impacts on future tropospheric ozone", American Geophysical Union (AGU) Fall meeting, San Francisco, U.S., 8-12 December, 2003.
Sudo, K., Akimoto, H., Nozawa, T., Kanzawa, H., and Takahashi, M., "Simulation of future distributions of tropospheric ozone and sulfate aerosol : impacts of emission change and climate change", International Conference on Earth System Modelling, Hamburg, Germany, 15-19 September, 2003.
Sudo, K., Takahashi, M., Nozawa, T., Kanzawa, H., and Akimoto, H., "Simulation of future distributions of tropospheric ozone and sulfate aerosol : impacts of emission change and climate change", International Union of Geodesy and Geophysics (IUGG), Sapporo, Japan, and 30 June - 11 July, 2003.
Kengo Sudo, Masaaki Takahashi, Hajime Akimoto, "The role and its change process of the stratosphere ozone in troposhere ozone chemistry", the 14th atmospheric-chemistry symposium, the Toyokawa citizen plaza, and 2004 Year One Moon 7-9 Day.
Kengo Sudo, Hajime Akimoto, Toru Nozawa, Hiroshi Kanzawa, Masaaki Takahashi, "A future prediction experiment of troposhere ozone and sulfuric-acid aerosol all ball distribution", and Meteorological Society of Japan 2003 [ Ten Moon 15-17 Day. ] A year autumn convention, the Miyagi resident hall, and 2003 Year Kengo Sudo, Hajime Akimoto, Masaaki Takahashi, Toru Nozawa, Hiroshi Kanzawa, "future prediction simulation of the troposhere photochemistry place using all the ball chemistry climate models CHASER", and the 9th time An atmospheric chemistry debate, Ikaho Hot Spring hotel Kogure, and 2003 Year Five Moon 28-30 Day.
Masayuki Takigawa, the "calculation of the stratosphere Age Spectral using CCSR/NIES general circulation model" 14th atmospheric-chemistry symposium, 2004. Responsibility for the wording of an article: Kengo Sudo (a., b., c., e-1., e-2., e-3., f.) Masayuki Takigawa (e-4., e-5.) Next Page (2.2 a warming-cloud, aerosol, and radiation feedback precision evaluation) |