2. Warming and atmospheric composition change interactionResults Page | Top Page | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
2-1 Warming and atmospheric composition change interaction (atmospheric chemistry) The organization in charge: Earth environment frontier research center
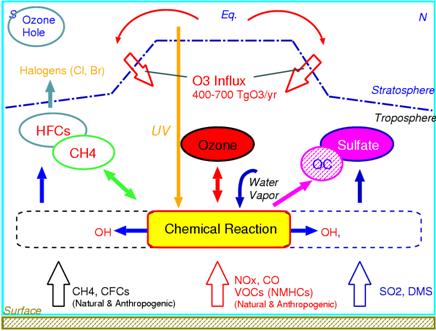
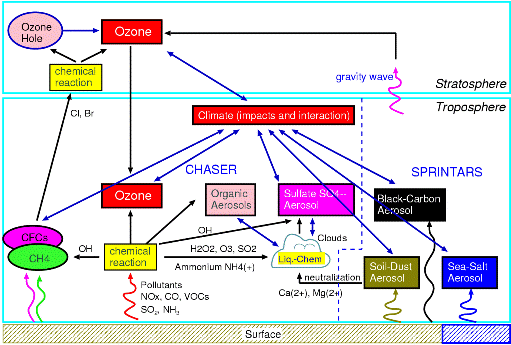
a. SummaryIn warming and an atmospheric composition change interaction sub model, it is a big subject to enable on-line calculation of aerosol and chemistry using all the ball chemistry models CHASER and the aerosol model SPRINTARS which set it as the main purposes to express and predict an interaction with warming of atmospheric chemistry process (ozone distribution etc.) or aerosol, and the ocean and terrestrial vegetation change, and used CCSR/NIES AGCM as the foundation. This fiscal year ended the substantial work which combines a CHASER model and a SPRINTARS model, the scheme especially combined with chemistry about the mode of expression of organic carbon aerosol generation of sulphate aerosol generation process and the vegetable origin was introduced, and improvement was performed. Furthermore, the work which builds the united CHASER-SPRINTARS component into an integrated model main part was done, and not only carbon (CO2) circulation but the on-line and the climate simulation included to chemistry and aerosol became possible. Moreover, the future prediction experiment of the ozone which followed IPCC/SRES each scenario using CHASER, methane, and sulphate aerosol was conducted on the Earth Simulator, and the difference in time development and the influence of a climate change according to each scenario about the obtained result were considered. Furthermore, it participated in the project of the atmospheric chemistry and air quality change relation in the 4th IPCC report as a CHASER model, and while performing reappearance / prediction calculation of a future past and present, and ozone place and a photochemistry place and contributing also to the mutual comparison between each model, influence of each variable factor (emission change, a climate change, the amount change of halogen) on an ozone place was also considered. b. Research purposeThe ozone (ozone layer) of the stratosphere is the existence which has the important role of intercepting harmful ultraviolet rays, and cannot be disregarded for a climate change. On the other hand, ozone is generated through a chemical reaction from contaminants, such as nitrogen oxide (NOx) and hydrocarbon, also all over the troposhere. The ozone in the troposhere is harmful to a plant and a human body, and importance is recognized as powerful greenhouse effect gas. The various aerosol of the troposhere also involves to sunlight reflection and absorption, and generation of clouds strongly, and influences both climates greatly. Moreover, ozone participates in generation of a hydroxylation radical (OH) directly in the troposhere, and influences the chemical life of other greenhouse effect gas, such as methane and halocarbons (CFCs), (Fig. 30). Furthermore, since troposhere ozone and sulphate aerosol hold substantially the key of atmospheric environment change represented by acid rain etc., it is important how these are changed by future human activities (especially East Asia region). Moreover, since there are some which have the chemistry of the troposhere, such as secondary organici matter aerosol (SOA) produced in the oxidization process of sulphate aerosol or hydrocarbon, and strong relevance in an aerosol kind, also in case future aerosol distribution and the influence on the climate are considered, it is necessary to use the model combined with the chemical process. Furthermore, the place where the chemical process of the troposhere is influenced by the conditions (climate) of weather places, such as steam, temperature, and circulating space, is large. (for example, Sudo et al., 2001, 2003) . Therefore, the model which can calculate ozone aerosol distribution simultaneously in the framework of a climate model in order to aim at more advanced climate change and atmospheric environment change prediction, and can examine an interaction change process of climate and ozone aerosol needs development and to be developed. In the framework of the bottom of such a background, and the 2nd subject of this symbiosis project The troposhere / stratosphere chemistry, and the climate model in which on-line calculation of aerosol is possible are built by using a CHASER-SPRINTARS car model as a foundation, and the interaction of chemistry and aerosol change, and a climate change (vegetation change is also included) is predicted and studied.
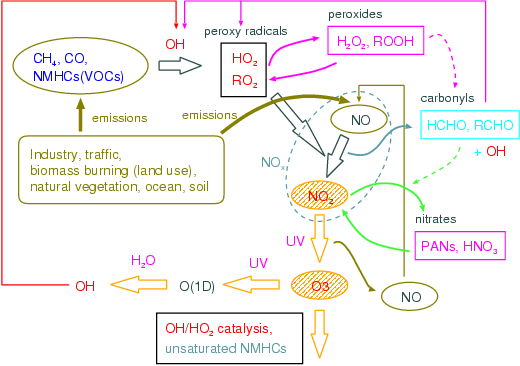
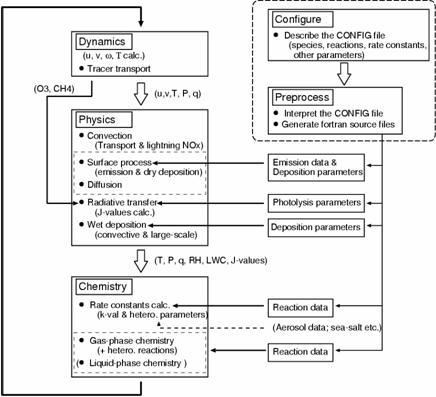
c. A research program, a method, a scheduleBy this subgroup research, they are all ball chemistry and a climate joint model. Model development and research centering on CHASER (Sudo et al., 2002a) are done. CHASER A model is the University of Tokyo climate system research center (CCSR), They are an earth frontier research system (FRSGC) and all the ball chemistry models currently developed jointly in National Institute for Environmental Studies (NIES), CCSR/NIES The chemical process with the photochemical reaction in the atmosphere, human work and natural origin outgassing (emission) process, the build-up (deposition) process by earth surface and precipitation, etc. detailed in a climate model is taken into consideration on-line (Table 3: : which is taking into consideration the chemical reaction system centering on troposhere ozone as a chemical reaction in the present setup figure 31). CHASER Ozone (O3) and precursor gases which are calculated by the model (nitrogen oxide NOx, carbon monoxide CO, hydrocarbon VOCs, etc.) And distribution of important related gas shows quantitive very good coincidence as the various observational data using a satellite or an airplane, and, also globally, goes a tip as simulation capability of troposhere ozone chemistry (Sudo et al., 2002b). Moreover, in a CHASER model, it is Fig. 32 That which generates a model code (Fortran) automatically by the Puri processor through a configuration file about chemical species or a chemical reaction system, and change and an addition of chemical species and a reaction are easy. This research program aims at the ozone chemical process of the troposhere and the stratosphere, and construction of the chemistry and the aerosol joint climate model in which the simultaneous simulation of various aerosol is possible by using a CHASER model as a foundation. Although all the ball chemistry models CHASER mainly target troposhere chemistry in a present condition setup, compared with standard AGCM, its calculation cost is very large including various chemical reactions. Then, in advance of the introduction of stratosphere chemistry and aerosol calculation including the ozone hole to CHASER, CHASER is accelerated (especially chemical process) and the execution performance on an Earth Simulator is evaluated. Moreover, after cooperating with a "climate physics core model improvement" subgroup, about the perpendicular resolution as an integrated model etc., he examines based on this evaluation and it is decided that it will be it (FY 2003). Furthermore, the work which combines the aerosol model SPRINTARS (Takemura et al., 2002) with the accelerated CHASER model is started. About the inside of an aerosol kind, and sulfuric acid aerosol, the generation process is hydrogen peroxide (H2O2), a hydroxylation radical (OH), and ozone.
In order to depend at chemistry places, such as distribution, strongly, on-line calculation is carried out in the chemical reaction process of CHASER (cf., Sudo, 2003). Under the present circumstances, the neutralization process by a soil particle (dust) or ammonia (NH3) is introduced also about the itinerant Buddhist pH important for oxidization by the liquid phase of sulphate aerosol, and a realistic sulphate simulation is realized (FY 2003 - 2004 ). Since especially the transport process in a model is important for the stratosphere / troposhere substance exchange and influence is large also to chemical substance distribution of the troposhere and the stratosphere, the evaluation and improvement of a transportation scheme which are used are also performed in parallel. Moreover, what introduced aerosol process into CHASER at the time of FY 2004 (first half) is included in an integrated model. The model top is extended after that and the work which introduces stratosphere chemistry into a CHASER chemical process is done (FY 2004 - 2005). About stratosphere chemistry introduction, the halogenation study reaction (chlorine and bromine system) in the stratosphere and the heterogeneous reaction on polar stratosphere clouds (PSCs) are introduced on the basis of the stratosphere chemistry and the ozone hole model (Takigawa et al., 1999; Nagashima et al., 2002) developed in CCSR and NIES. In the framework of an integrated model, it assumes also expressing the interaction between a terrestrial ecosystem and atmospheric chemistry in consideration of the influence on the discharge process to the inside of the atmosphere of VOCs from vegetation and the self-possessed process (deposition) of the substance in the atmosphere by vegetation, and the vegetation according to the build-up of substances, such as nitric acid, further. In parallel to the above model construction work, the preceding paragraph story-experiment for joint future prediction of atmospheric chemistry and aerosol change, and warming is also conducted. For example, CHASER is used. IPCC SRES The future prediction experiment according to a scenario is started, distribution of future ozone methane or sulphate aerosol It is analyzing about what kind of effect the increase in emission (especially East Asia region) and warming of contaminants, such as NOx, CO, VOCs, and SO2, have, respectively (about the influence by warming, the ozone destruction of a troposhere lower layer promotes by the increase in steam -- having -- simultaneous -- OH from ozone -- there are possibilities, such as radical generation increasing and affecting the upward tendency of methane). The outline-schedule of the model development of this subgroup is shown in Fig. 33.
Fig. 32: Flow of CHASER model calculation. It is calculated on-line in CCSR/NIES AGCM about dynamics (transportation is included), a physical process, and each chemical process. A setup of chemical processes, such as a chemical reaction and addition of chemical species, is performed through a configuration file, and Fortran source code is generated automatically (cf., Sudo, 2003).
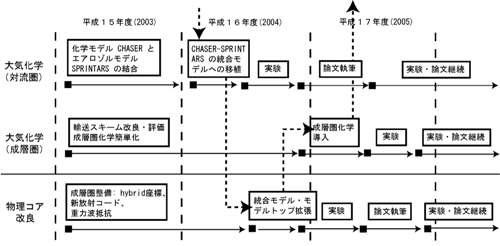
Fig. 33: Development work schedule of warming and atmospheric composition change interaction subgroup. A dotted line shows the flow of K2 integrated model development. d. The research program in FY 2004Various aerosol process is introduced into chemistry and the climate model CHASER (it develops on the University of Tokyo climate center and an earth frontier), and the model which can simultaneous evaluate the warming influence of ozone methane aerosol change (interaction) is built. As an aerosol process, it sets on the basis of a SPRINTARS model (the University of Tokyo climate center / Research Institute for Applied Mechanics , Kyushu University ), and relation with chemistry combines with the chemical reaction process of CHASER about a strong sulphate or organic carbon aerosol. Moreover, CHASER is transplanted to an integrated model after aerosol introduction. Furthermore, with the improvement of an advection current and physical process each scheme, the stratosphere chemistry (halogen and PSCs reaction) introduction to CHASER is started. e. Fruits of work in FY 2004e.1. CHASER, combination of a SPRINTARS car model and introduction to an integrated model The work which introduces various aerosol simulations into all the ball chemistry models CHASER in the framework of the 2nd integrated modeling of symbiosis was completed. As aerosol related process SPRINTARS Although based on the model, it is related with sulphate aerosol or a part of organic carbon aerosol (secondary generation organici-matter aerosol: SOA). CHASER It calculates inside. It is especially sulphate aerosol. (SO42-) In the generation process in the inside of an itinerant Buddhist, the neutralization process by cations, such as a soil particle (calcium2+, Fe3+, etc.) and ammonium (NH4+), is also taken into consideration, It linked with the chemistry place, and it depended and the realistic simulation was made possible (since it seems that fault is still in a code at present although a thermodynamic equilibrium model is introduced as a simulation of * ammonium and the equilibrium situation of the vapor phase / aerosol phase in the system of nitric acid and ammonium sulfate is expressed, it calculates by accepting it in a tentative way). About the chemistry and the aerosol-sub model which built, a test experiment is conducted on an Earth Simulator, calculation performance is evaluated, and the calculation result is also checking as compared with the thing of chemistry and an aerosol simplex model. Furthermore, the work which introduces this sub model into an integrated model main part is done, and the atmosphere and a sea simulation not only including CO2 circulation but chemistry and aerosol process are possible as an integrated model at present. Fig. 34 shows the summary of the chemistry and the aerosol modeling in this integrated model.
Fig. 34: Modeling of atmospheric chemistry and aerosol process in framework of symbiosis and earth system integrated model. It is put into the influence of the radiation process on greenhouse effect gas, such as ozone methane, and the influence of the cloud process on an aerosol kind by consideration including a detailed chemical reaction process and aerosol calculation.
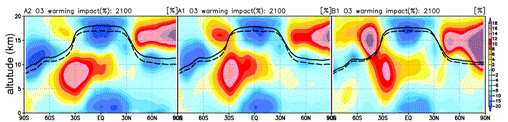
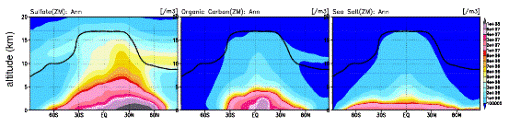
Fig. 35: Annual average east-and-west average number density distribution of various aerosol calculated by K2 integrated model (m-3). A sulphate (left), organic carbon aerosol (inside), sea salt (right). The approvement is performed about the generation process in the liquid phase of sulfate aerosol as mentioned above, and easy parameterization using the oxidation reaction by the ozone of terpenes is introduced in a tentative way about generation of the carbon nature aerosol from the vegetable origin (terpenes are assumed). The concentration of each aerosol kind is reflected also in each process of the photodissociation by the ultraviolet and visible light of gaseous phase chemical species, and a heterogeneous reaction (reaction through an aerosol particle face) on-line, and is also expressing the reaction on the atmospheric chemistry which aerosol does at the same time it is reflected in a radiation field and large-scale condensation (cloud and precip) process in the framework of a climate model. Furthermore, about the dry deposition in the earth surface of gaseous phase chemical species, partial combination with land process was performed using the stomatal resistance in the land model MATSIRO of an integrated model. About combination with atmospheric chemistry and land process, it is planning not only dry deposition but expressing emission of vegetable origin hydrocarbon on-line using MATSIRO/SimCYCLE/DGVM as a plot of next year (FY2005). The test experiment was conducted on the Earth Simulator in the second half of this fiscal year about this integrated model into which atmospheric chemistry and aerosol were introduced as mentioned above, comparison with the result especially depended on a CHASER/SPRINTARS car model simple substance about the result of chemistry and aerosol relation was performed, and adjustment was checked. Although it was checked that the same simulation as the case where it calculates with a CHASER simple substance about the result of the chemical-species relation calculated by the CHASER component as a result can be performed, it checked that the case where it calculates with a SPRINTARS simple substance about the output (aerosol concentration distribution, optical thickness, and number density-CCN distribution) of a part of aerosol relation, and a significant difference might be seen. In the integrated model developed about sulphate aerosol this time, calculating within CHASER is the big cause and especially this has calculated the especially larger sulphates in the Nakagami part troposhere as compared with SPRINTARS (east-and-west average advanced (as ideal CCN) distribution of the aerosol number density calculated by the integrated model this time is shown in Fig. 35 as an example). Since adjustment with vapor phase chemical species is taken about wet deposition process about other aerosol kinds, it is made to calculate by the wet deposition scheme in CHASER, and a difference may be produced a little compared with SPRINTARS, but fundamental distribution pattern and quantity are near. It is necessary to evaluate in more detail about the amount of aerosol from now on, and the parameter tuning of chemistry and aerosol process or radiation, and the re-tuning by the parameter tuning of large-scale condensation process may also be needed. In the examination RUN of this integrated model main part, model resolution was set to T42L32 and used Earth Simulator L system 4 node (air 2+ ocean 2). The execution time at the time of one-year integration was 4.5 hr/year, and the average items of air node time were 42% of chemical processes, 28% (a transport process is included) of dynamics process, 8% of radiation process, and 9% of communications between nodes. The rate of mean-vector-izing was -97%. e-2. The future prediction experiment according to IPCC each scenario IPCC/SRES According to A2, A1, and B1 each scenario, the future prediction experiment of troposhere ozone, methane, and sulphate aerosol all ball distribution was conducted. The experiment was performed on the Earth Simulator (L system) using the chemical bond climate model CHASER. This prediction experiment puts emphasis on the influence of the chemistry place on a climate change like the experiment in the last fiscal year, analyzed the influence which change of steam, temperature, and an atmospheric circulation place has on ozone methane and sulphate aerosol distribution, or the stratosphere / substance (ozone) exchange between the troposhere in addition to a future emission change, and evaluated in detail also about the difference between each scenario. It had cooperated also in the climate change prediction experiment whose research of this the symbiosis first division title (K-1) is doing towards the fourth IPCC report (AR4), and the prediction data of the ozone methane distribution by this experiment was offered as an input value of K-1 experiment. In this investigation, two kinds of experiments, the experiment (Exp1) of only dishoarding (emission) diversification of ozone, such as nitrogen oxide NOx and carbon monoxide CO, and precursor gases and the experiment (Exp2) which also took the climate change into consideration in addition to emission diversification, were performed. In these experiments, a future emission change and a climate change gave the sea surface temperature (SSTs) and sea ice (Sea-ice) distribution which were predicted by the CCSR/NIES air sea joint model about each scenario while making greenhouse effect gas concentration, such as CO2, both increase in a model about the climate change of Exp2 according to each scenario of A2 (increase size in emission), A1 (this Naka), and B1 (this smallness) of IPCC SRES. Fig. 36 is the amount distribution of increases of the ozone near the surface of the earth from the present condition (let 2000 be a base) calculated by Exp1 (the present climate without warming) to 2100, and a big difference is seen between each scenario. In A2 scenario supposing the increase in emission almost linear till 2100, the increase in ozone is especially calculated globally, and the big increase in surface-of-the-earth ozone of 10 or more ppbv is calculated also on 30 or more ppbv and the Northern Hemisphere ocean in the East Asia region. Although emission reduction is assumed in North America, Europe, Australia, Japan, etc. and it is calculated in 2100 of A1 scenario that surface-of-the-earth ozone also decreases a little (2-4ppbv) compared with the present condition in connection with this, It seems that it is influenced by background by the increase in emission in other domains (for example, East Asia and India) of the increase in ozone (Japan is the increase in ozone in response to the Chinese influence of the increase in emission, although emission decreases). In South America, Africa, India, and China, it is the increase in ozone of 10 or more ppbv.
Fig. 36: Amount (ppbv) of annual average ozone diversification in 2000 to 2100 calculated by CHASER. IPCC-SRES A2/A1/B1 When each scenario is used (the experiment without a climate change: Exp1).
Fig. 37: Influence future warming affects ozone place (east-and-west average) (%) (calculation in 2100 according to A2, A1, and B1 each scenario). The difference of Exp2 and Exp1 defines warming influence. Dashed line: The 2000 tropopause altitude, the 2100 tropopause altitude of solid line:each scenario warming experiment (Exp2).
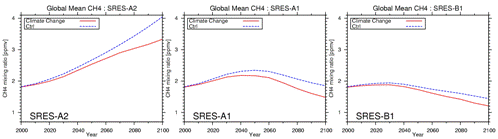
Fig. 38: Time development of total ball average methane concentration (ppmv) calculated according to SRES each scenario. Solid line: A warming experiment (Exp2), a dashed line:present climate experiment (Exp1) On the other hand in B1 scenario which assumes about 20% of emission abatement in 2100 compared with status quo, it becomes surface-of-the-earth ozone abatement almost globally, and ozone abatement of 5-10ppbv is calculated in the Northern Hemisphere deterioration region (in status quo). Although the experiment (Sudo et al., 2003) conducted in the last fiscal year considered the influence future warming affects ozone distribution only about A2 scenario, this experiment estimated not only A2 but A1 and B1. It is shown what kind of difference comes out of Fig. 37 to east-and-west average ozone distribution by the case (Exp1) where warming is also taken into consideration about 2100 of each scenario in addition to the case (Exp1) of only emission change and emission change. Although 10-20% of reaction of negative is calculated to ozone concentration in the troposhere lower layer (especially tropical region) with an altitude of 5km or less in A2 and A1 scenario with the large proceeding of warming, it reflects that ozone came to be easily destroyed in photochemistry in connection with warming by the increase in steam. Conversely, in the Nakagami part troposhere Torrid Zone region, about 10% of ozone level rise is especially seen, and it is remarkable in the Southern Hemisphere. It is especially the result of changing the general circulation of the atmosphere sharply about this by a climate change (warming in the troposhere) as well as the experiment conducted in the last fiscal year, and originates in the ozone inflow from the stratosphere having increased notably by having strengthened the circulation (Brewer-Dobson circulation) in the stratosphere. By the tropical region lower layer area, the increase in downward flow takes place by strengthening of stratosphere circulation in the increase in upward flow, and the lower stratosphere of inside and high latitude, and near the tropical region tropopause, increase descent is calculated by the reduction effect of an ozone level, and inside and high latitude so that it may correspond to these. Although reduction in an ozone level is seen also near the tropopause of inside and high latitude, this is reflecting that the tropopause altitude rose by warming as shown in a figure. Moreover, the same influence as A2 and A1 is calculated, and B1 scenario with the comparatively small degree of warming (climate change) is also interesting. Future warming affects other substance and processes greatly not only through ozone distribution but through a photochemical reaction. For example, time development of the total ball average methane concentration predicted in this future experiment is shown in Fig. 38. First, since emission of the methane which changed with scenarios is assumed if it takes notice of the experiment (Exp1: dotted line) which does not take warming into consideration, it turns out that the methods of time development of methane concentration differ greatly. In A1 and B1 scenario with which increasing from the present concentration (~1.8ppm) greatly in the case of A2 scenario, and increasing to it to near the 4ppmv by 2100 is calculated, and it assumes the emission reduction from the middle of the 21st century to be, becoming the same grade as the present condition or a value lower than it is predicted in 2100. Thus, although time growth which changed with methods of emission diversification is carried out, when warming is taken into consideration about every scenario, it turns out that there is cost performance of 20-30% of remarkable methane concentration abatement. This is reflecting that the chemical reaction which destroys methane by the increase in a water vapour content and rise in temperature accompanying warming of the troposhere activates. e-3. Participation to the 4th IPCC report (AR4) related project (atmospheric chemistry) It is the IPCC-4th report AR4 in the second half of this fiscal year. Section 7.3/7.4 Since it contributed to the mutual comparison project of the past, the present, and the future experiment by each model etc. in "Global Atmospheric Chemistry and Climate Change", the various scenario experiments about ozone, methane, and a sulphate were conducted using CHASER and an Earth Simulator, and the calculation result was submitted about each project. It divides roughly, and is divided into two kinds of experiments, and these projects are the Industrial Revolution or before and the present by the 1st experiment (Exp1),About each quantity of the present and the future (here, 2100 are assumed), it is ozone precursor gases emission diversification,Importance sets on how a climate change and concentration change of halogen chemical species influence ozone distribution, some kinds of experiments to which the parameter was changed about various factors are conducted, and it argues about the reaction about each factor (Table 4). It aims at the detailed mutual comparison about the atmospheric chemistry place simulation centering on the ozone of the present condition (it will be an object about 2000), and the future (- 2030 object) in the second experiment (Exp2) (Table 5). It is related especially with a future prediction experiment, and is IPCC SRES-A2 by experiment 1. To assuming 2100 of a scenario, in the experiment 2, it depends also including atmospheric environment and vegetation influence for - 2030, and emphasis is put on realistic prediction and its mutual comparison between models. Having participated in this project are 12 models by Europe, the U.S., and Japan to the experiment 1, In the experiment 2, it is 24 models and, in a NCAR-WACM model (NCAR), MOZART (University of Illinois), GISS, GEOS-CHEM (Harvard University), etc., ULAQ (Italy), UIO (Norway: Oslo University), LMDzINCA (France: CNRS/CEA), DLR (Germany: DLR) and TM 4/5 (Netherlands: KNMI), STOCHEM/UM_CAM/STOCED/SLIMCAT/TOMCAT (Britain: UK_Met, Cambridge University, Edinburgh University, etc.), etc. have mainly participated from the U.S. from Europe. From Japan, 2 of CHASER (JAMSTEC/FRCGC) and a FRSGC_UCI model (JAMSTEC/FRCGC) models participated. Table 4: Scenario setup in experiment 1 of IPCC-AR4 (atmospheric chemistry) project
Table 5: Scenario setup in experiment 1 of IPCC-AR4 (atmospheric chemistry) project (at CHASER, all the following scenarios are performed in the mode of two kinds of CTM/GCM)
Fig. 39 shows change of the ozone level place (east-and-west average) from the Industrial Revolution or before calculated by CHASER about the project of experiment 1 to the present, and the influence of each factor as an example of experimental findings. As ozone change of a network, the big increase in ozone of 10 or more ppbv is calculated by troposhere average reflecting the increase in precursor gases emission (the remarkable increase in ozone which attains to -20ppbv is seen especially in the Northern Hemisphere Nakagami part troposhere), and the remarkable ozone phenomenon centering on the South Pole ozone hole is checked conversely in the stratosphere. If it divides into each component, the reaction of emission ozone precursor gases serves as increase in ozone of about 20 ppbv throughout the Northern Hemisphere most greatly. Although the effect of stratosphere ozone reduction has the influence fundamentally limited in the stratosphere, the influence which it has on the ozone distribution in the troposhere cannot disregard it, either. Moreover, it turns out that ozone change (ozone reduction of a tropical region and increase in ozone of inside latitude) of the lower stratosphere accompanying the ozone reduction and the stratosphere cyclical variation accompanying the increase in steam of the tropical region lower troposhere etc. is calculated as influence which a climate change has, and these cannot be disregarded, either. Same calculation is performed also to the ozone change from the present to the future (2100 at the time of using SRES-A2 scenario) (Fig. 40), Also when predicting change of future ozone distribution, the necessity for taking not only diversification of precursor gases emission but the change (change of amount of halogen chemical species) process of a climate change (warming) and stratosphere ozone into consideration, and predicting and evaluating it synthetically like the Industrial Revolution or before and the present, was suggested. It is shown by Fig. 40 that it is effective in the ozone concentration of a troposhere lower layer decreasing by warming like that the ozone concentration of the Nakagami board troposhere rises further under a rally of a stratosphere ozone layer and the reaction of a climate change or the argument of Fig. 37. Therefore, when discussing the climate influence of troposhere ozone (radiation legal force) conventionally, only change of emission of precursor gases was taken into consideration, but it is suggested that stratosphere ozone change and a climate change should also be taken into consideration, and ozone change and radiation legal force should be evaluated (schedule which has calculated radiation legal force using the ozone level place submitted by the person in charge of the present project about each model and each scenario, and is reflected in IPCC-AR4). On the other hand, in experiment 2, about prediction, the more realistic prediction experiment will be aimed at in the future compared with the experiment 1 for 2030, and it is arguing about the influence of the climate change by 2030 as well as experiment 1 about emission of ozone precursor gases supposing four kinds (Table 5). Ozone is evaluating by the experiment 2 including the mutual comparison between models also about prediction of environmental sides, such as influence which it has on surface-of-the-earth vegetation, and a build-up (acid rain) of acid things. Furthermore, in the experiment 2, comparison with a observation and mutual comparison between models are performed about each model also with the simulation of the atmospheric chemistry place of not only a prediction experiment but status quo (- 2000). Fig. 41 compares with the satellite observation by GOME as an example the amount distribution of troposhere columns of the nitrogen peroxide NO2 (being a part of NOx chemical species important for ozone generation) calculated by CHASER. In addition, comparison with observation and mutual comparison between models are performed by each model also about ozone distribution and various self-possessed quantity distribution.
Fig. 39: Influence by east-and-west average ozone change (a) by the present before the Industrial Revolution calculated by CHASER -, and each factor (ozone precursor gases emission change, c:stratosphere ozone change, d: climate change). Solid line: (IPCC-AR4 related project experiment 1) The tropopause altitude of the present (2000), the tropopause altitude of the climate before the dashed line:Industrial Revolution.
Fig. 40: Influence by east-and-west average ozone change (a) by the present - 2100 and each factor which were calculated by CHASER (ozone precursor gases emission change, c:stratosphere ozone change, d: climate change). (IPCC-AR4 related project experiment 1) A scenario is based on SRES-A2. Solid line: The tropopause altitude in 2100, the tropopause altitude of dashed line:present climate.
Fig. 41: Comparison with amount of NO2 columns (1015molecules cm-2) and satellite data (GOME) which were calculated by CHASER. (IPCC-AR4 related project experiment 2) . f. ConsiderationIn the work in FY 2004, first, the chemistry climate model CHASER and the aerosol climate model SPRINTARS were combined, and inclusion on an integrated model main part was also ended. Especially in this work, a chemistry place and expression made to link were introduced about the liquid phase generation process of sulphate aerosol, and the more realistic sulphate aerosol simulation became possible. However, since it differs from the thing of a SPRINTARS simple substance in part about the mode of expression of the various aerosol containing sulphate aerosol, between the aerosol distribution calculated by the integrated model built this time, and the distribution calculated by the conventional SPRINTARS, it is checking that there may be a significant difference. Since it is thought that the influence on climate sensitivity of calculation of aerosol concentration is also large, while continuing detailed evaluation of the aerosol calculation in an integrated model, examination of readjustment (tuning) is also required including radiation process and large-scale condensation (cloud/precipitation) process. Although the calculation which made it depend for a part of dry deposition of vapor phase chemical species on the land model MATSIRO was introduced by the work in FY 2004 about combination with atmospheric chemistry and earth surface process, on the other hand, it is also under examination further the emission process of the hydrocarbon from vegetation, the influence process to the vegetation of ozone, the emission process of DMS from the ocean, and to calculate on-line from now on. The future prediction experiment of an ozone chemistry place was conducted about three scenarios of IPCC-SRES, and it provided as an input of an IPCC climate experiment of a symbiosis first division title about the result in FY 2004. The influence which future warming has on ozone methane distribution from this future experiment is remarkable when every scenario is followed, and it has suggested that it is necessary to take into consideration the interaction of atmospheric chemistry and climate in future prediction of climate and atmospheric environment. furthermore, although this year participated in the atmospheric chemistry-related project of the 4th IPCC account using the CHASER model and the scenario experiment about - future be conducted the past and now, in this experiment, the conclusion that it be necessary to evaluate simultaneously not only emission diversification of ozone precursor gases but change of stratosphere ozone and a climate change in the change process of related chemical species, such as an ozone concentration place and methane, be obtained. After next year, the stratosphere chemical process based on a CCSR/NIES stratosphere chemistry model (Takigawa et al., 1999, 2001, Nagashima et al., 2002, Akiyoshi et al., 2002) is introduced into a CHASER component after that the radiation process of an integrated model main part, and perpendicular coordinates and resolution improve. Under the present circumstances, if possible, introduction of an improvement scheme (Kurokawa et al., 2002, Nagashima et al., 2003) will be considered also about the surface-of-a-sphere effect in radiation process. g. Bibliography(1) Akiyoshi H., T. Sugata. S. Sugita, H. Nakajima, H. Hayashi, J. Kurokawa, M. Takahashi, Lower-N2O air masses after the breakdown of the arctic polar vortex in 1997 simulated by the CCSR/NIES nudging CTM, J. Meteorol. Soc. Jpn, 80, 451-463, 2002. (2) Nagashima, T., M. Takahashi, M. Takigawa, and H. Akiyoshi, Future development of the ozone layer calculated by a general circulation model with fully interactive chemistry, Geophys. Res. Lett., 29, 10.1029/2001GL014926, 2002. (3) Nagashima, T., M. Takahashi, H. Akiyoshi, and M. Takigawa, The effects of non-orographic GWD scheme and radiation from large SZA on the Antarctic ozone hole, Process-oriented validation of coupled chemistry-climate models, 2003. (4) Sudo, K. and M. Takahashi, Simulation of tropospheric ozone changes during 1997-1998 El Nino: Meteorological impcat on tropospheric photochemistry, Geophys. Res. Letters., 28, 4091-4094, 2001. (5) Sudo, K., M. Takahashi, J. Kurokawa, and H. Akimoto, CHASER: A global chemical model of the troposphere 1. Model description, J. Geophys. Res., 107, 10.1029/2001JD001113, 2002a. (6) Sudo, K., M. Takahashi, and H. Akimoto, CHASER: A global chemical model of the troposphere 2. Model results and evaluation, J. Geophys. Res., 107, 10.1029/2001/JD001114, 2002b. (7) Sudo, K., Changing process of global tropospheric ozone distribution and related chemistry: a study with a coupled chemistry GCM, Doctoral Dissertation, Dept. of Earth and Planetary Science, Graduate School of Science, Univ. of Tokyo, 187pp, 2003. (8) Sudo, K., M.Takahashi, and H.Akimoto, "Future changes in stratosphere-troposphere exchange and their impacts on future tropospheric ozone simulations", Geophysical Research Letters., 30, 24, 2256, doi:10.1029/2003GL018526, 2003. (9) Takemura, T., T. Nakajima, O. Dubovik, B.N. Holben, and S. Kinne, Single-Scattering albedo and radiative forcing of various aerosol species with a global three-dimensional model, Journal of Climate, 15, 4, 333-352, 2002. (10) Takigawa, M., M. Takahashi, and H. Akiyoshi, Simulation of ozone and other chemical species using a center for climate system research/national institute for environmental studies atmospheric GCM with coupled stratospheric chemistry, J. Geophys. Res., 104, 14,003-14,018, 1999. (11) Takigawa, M., M. Takahashi, and H. Akiyoshi, Simulation of stratospheric sulfuric acid aerosol using a Center for Climate System Research / National Institute for Environmental Studies atmospheric GCM with coupled stratospheric chemistry: Part I, nonvolcanic simulation, J. Geophys. Res., doi: 10.1029/2001JD001007, 2002. (12) Junichi Kurokawa, Eiji Akiyoshi, Tatsuya Nagashima, Hideaki Nakane, Hirohiko Masunaga, Teruyuki Nakajima, and Masaaki Takahashi : the collection of an operative induction and the air surface-of-a-sphere Meteorological Society of Japan spring rally lecture drafts in the 2002 fiscal year to a CCSR/NIES stratosphere nudging chemistry transportation model and a photochemistry joint model, and p -- 346 and 2002. h. The announcement of a result<Paper announcement> (1) M.G. Laurence, O.Hov, M.Beelamann, J. Brandt, H. Elbern, H.Eskes, H.Feichter, and M.Takigawa, <Oral announcement> (1) Sudo, K., Takahashi, M., and Akimoto, H., "The roles of stratospheric ozone in past and future tropospheric ozone budgets and trends", Quadrennial Ozone Symposium QOS 2004, Kos Greece, 1-9 June, 2004. (2) Sudo, K., Takahashi, M., Nozawa, T., Kurokawa, J., and Akimoto, H., "Tropospheric ozone trends and budgets during 1850-2000 simulated in a chemistry coupled climate model", 8 th International Global Atmospheric Chemistry Conference, Christchurch, New Zealand, 4-9 September, 2004. (3) Sudo, K., Takigawa., M., Takahashi, M., "Chemistry and aerosol modeling in the Kyousei -2 : toward understanding chemistry/climate interaction", Workshop on Climate Change Research, Yokohama Japan, 28-29 October, 2004. (4) Sudo, K., "Atmospheric chemistry-climate interaction", Third Japan-EU Workshop on Climate Change Research, Yokohama Japan, 20-21 January, 2005. (5) Takigawa, M, and H.Akimoto, and M.Masaaki, "Evaluation of transport in CCSR/NIES AGCM by using age spectra", Ozone Symposium 2004, Kos, Greece, 1-8 June, 2004. (6) Takigawa, M, and M.Takahashi, and H.Akiyoshi, "Simulation of stratospheric sulfur aerosol using a CCSR/NIES AGCM with coupled chemistry : The impact of Pinatubo aerosol on climate", KAGI (7) Kengo Sudo, Hajime Akimoto, Masaaki Takahashi, "The influence of the troposhere ozone and OH radical concentration place on a plant / artificial origin methanol", and Meteorological Society of Japan 2004 [ Five Moon 16-19 Day. ] A year spring convention, the Meteorological Agency, and 2004 Year (8 Kengo Sudo, Hajime Akimoto, Masaaki Takahashi, "the influence of the troposhere ozone and OH radical concentration place on a plant / artificial origin methanol", and the 10th time An atmospheric chemistry debate, the University of Tokyo and Research Center for Advanced Science and Technology, and 2004 Year Six Moon 23-25 Day. (9) Kengo Sudo and Hajime Akimoto, Masaaki Takahashi, "Where the separation:contaminant by each sauce region of troposhere O3 and all CO ball distribution flows how much from", and Meteorological Society of Japan A 2004 year autumn convention, acrose Fukuoka, and 2004 year 10 moon 6-8 day. (10) Kengo Sudo, Development and its application of "chemistry and a climate joint model: The change process of troposhere ozone chemistry, chemistry and climate interaction", Meteorological Society of Japan 2004 [ Ten Moon 6-8 Day. ] A year autumn convention, acrose Fukuoka, and 2004 Year (11) Kengo Sudo, Masaaki Takahashi, Hajime Akimoto, The past / present / future of "troposhere ozone chemistry, aerosol, and climate: The chemistry and climate joint simulation" towards the 4th IPCC report, the 15th atmospheric-chemistry symposium, the Toyokawa citizen plaza, and 2005 Year One Moon 5-7 Day. (12) Kengo Sudo and Hajime AkimotoA Masaaki Takahashi, "Where the separation:contaminant by each sauce region of troposhere O3 and all CO ball distribution flows how much from", the 15th atmospheric-chemistry symposium, the Toyokawa citizen plaza, and 2005 year 1 moon 5-7 day. (13) Masayuki Takigawa, Kengo Sudo, Hajime AkimotoA Masaaki Takahashi, "the influence of the long haul during an aircraft observation campaign PEACE-C period", the 15th atmospheric-chemistry symposium, Toyokawa, 1 / 5-7 2005. Next Page (2.2 a warming-cloud, aerosol, and radiation feedback precision evaluation) |
![Fig. 36: Amount (ppbv) IPCC-SRES A2 of annual average ozone change during [ it was calculated by CHASER ] 2000 to 2100 / A1/B1 When each scenario is used (the experiment without a climate change: Exp1)](./figure/2004-36.gif)