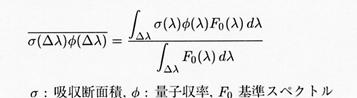2. Warming and atmospheric composition change interactionResults Page | Top Page |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
2-1 Warming and atmospheric composition change interaction (atmospheric chemistry)The organization in charge: Frontier Research Center for Global Change
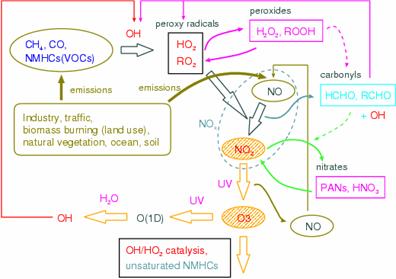
a. SummaryIn warming and an atmospheric composition change interaction sub model, it is a big subject to enable on-line calculation of aerosol and chemistry using all the ball chemistry models CHASER and the aerosol model SPRINTARS which set it as the main purposes to express and predict an interaction with warming of atmospheric chemistry process (ozone distribution etc.) or aerosol, and the ocean and terrestrial vegetation change, and used CCSR/NIES AGCM as the foundation. Stratosphere ozone change process was first introduced into the CHASER model in simple, and the future prediction experiment of all ball ozone level places was conducted in H17 fiscal year (it is hereafter described as this fiscal year) according to IPCC-SRES each scenario. Although the influence future warming affects atmospheric chemistry process was becoming clear in the experiment conducted by H16 fiscal year (it is hereafter described as the last fiscal year), it analyzed from this experiment about what kind of influence change of the stratosphere ozone of further the future has. It understood that especially recovery of the future amount of stratosphere ozone influences greatly the stratosphere / exchange between the troposhere of ozone (STE), and makes all the ball total amounts of troposhere ozone increase to it in every scenario. On the other hand, it checked that stratosphere ozone change taken into consideration this time hardly affected methane and sulfuric acid aerosol concentration of a total ball average. Moreover, a CHASER model is used from the last fiscal year. Although the experiment which contributes to the 4th IPCC report is conducted, analysis is advanced contacting Read Orser etc. succeedingly also about this. This fiscal year did the work which introduces a stratosphere chemical process into a CHASER model further, and performed introduction of numerical orientation method construction and improvement of the photodissociation constant for stratosphere chemistry, and a stratosphere chemical reaction, and maintenance of emission data. The chemical species taken into consideration in CHASER by this work corresponding to stratosphere chemistry became 79 kinds, and the chemical reaction became 213 (the heterogeneous reaction in the stratosphere is not included). b. Research purposeThe ozone (ozone layer) of the stratosphere is the existence which has the important role of intercepting harmful ultraviolet rays, and cannot be disregarded for a climate change. On the other hand, ozone is generated through a chemical reaction from contaminants, such as nitrogen oxide (NOx) and hydrocarbon, also all over the troposhere. The ozone in the troposhere is harmful to a plant and a human body, and importance is recognized as powerful greenhouse effect gas. The various aerosol of the troposhere also involves to sunlight reflection and absorption, and generation of clouds strongly, and influences both climates greatly. Moreover, ozone participates in generation of a hydroxylation radical (OH) directly in the troposhere, and influences the chemical life of other greenhouse effect gas, such as methane and halocarbons (CFCs), (Fig. 21). Furthermore, since troposhere ozone and sulphate aerosol hold substantially the key of atmospheric environment change represented by acid rain etc., it is important how these are changed by future human activities (especially East Asia region). Moreover, since there are some which have the chemistry of the troposhere, such as secondary organici matter aerosol (SOA) produced in the oxidization process of sulphate aerosol or hydrocarbon, and strong relevance in an aerosol kind, also in case future aerosol distribution and the influence on the climate are considered, it is necessary to use the model combined with the chemical process. Furthermore, the place where the chemical process of the troposhere is influenced by the conditions (climate) of weather places, such as steam, temperature, and circulating space, is large. (for example, Sudo et al., 2001, 2003) . Therefore, the model which can calculate ozone aerosol distribution simultaneously in the framework of a climate model in order to aim at more advanced climate change and atmospheric environment change prediction, and can examine an interaction change process of climate and ozone aerosol needs development and to be developed. In the framework of the bottom of such a background, and the 2nd subject of this symbiosis project The troposhere / stratosphere chemistry, and the climate model in which on-line calculation of aerosol is possible are built by using a CHASER-SPRINTARS car model as a foundation, and the interaction of chemistry and aerosol change, and a climate change (vegetation change is also included) is predicted and studied.
c. A research program, a method, a scheduleBy this subgroup research, they are all ball chemistry and a climate joint model. Model development and research centering on CHASER (Sudo et al., 2002a) are done. CHASER A model is the University of Tokyo climate system research center (CCSR), They are an earth frontier research system (FRSGC) and all the ball chemistry models currently developed jointly in National Institute for Environmental Studies (NIES), CCSR/NIES The chemical process with the photochemical reaction in the atmosphere, human work and natural origin outgassing (emission) process, the build-up (deposition) process by earth surface and precipitation, etc. detailed in a climate model is taken into consideration on-line (Table 2: : which is taking into consideration the chemical reaction system centering on troposhere ozone as a chemical reaction in the present setup figure 22). CHASER Ozone (O3) and precursor gases which are calculated by the model (nitrogen oxide NOx, carbon monoxide CO, hydrocarbon VOCs, etc.) And distribution of important related gas shows quantitive very good coincidence as the various observational data using a satellite or an airplane, and, also globally, goes a tip as simulation capability of troposhere ozone chemistry (Sudo et al., 2002b). Moreover, in a CHASER model, it is Fig. 23 That which generates a model code (Fortran) automatically by the Puri processor through a configuration file about chemical species or a chemical reaction system, and change and an addition of chemical species and a reaction are easy. This research program aims at the ozone chemical process of the troposhere and the stratosphere, and construction of the chemistry and the aerosol joint climate model in which the simultaneous simulation of various aerosol is possible by using a CHASER model as a foundation. Although all the ball chemistry models CHASER mainly target troposhere chemistry in a present condition setup, compared with standard AGCM, its calculation cost is very large including various chemical reactions. Then, in advance of the introduction of stratosphere chemistry and aerosol calculation including the ozone hole to CHASER, CHASER is accelerated (especially chemical process) and the execution performance on an Earth Simulator is evaluated. Moreover, after cooperating with a "climate physics core model improvement" subgroup, about the perpendicular resolution as an integrated model etc., he examines based on this evaluation and it is decided that it will be it (the Heisei 15 fiscal year). Furthermore, the work which combines the aerosol model SPRINTARS (Takemura et al., 2002) with the accelerated CHASER model is started. In order that generation process may depend for sulfuric acid aerosol at chemistry places, such as "hydrogen peroxide (H2O2) hydroxylation radical (OH)" and ozone distribution, strongly among aerosol kinds, on-line calculation is carried out in the chemical reaction process of CHASER (cf., Sudo, 2003). Under the present circumstances, the neutralization process by a soil particle (dust) or ammonia (NH3) is introduced also about the itinerant Buddhist pH important for oxidization by the liquid phase of sulphate aerosol, and a realistic sulphate simulation is realized (the Heisei 15 - 16 fiscal year). Since especially the transport process in a model is important for the stratosphere / troposhere substance exchange and influence is large also to chemical substance distribution of the troposhere and the stratosphere, the evaluation and improvement of a transportation scheme which are used are also performed in parallel. Moreover, what introduced aerosol process into CHASER at the time of the Heisei 16 fiscal year (first half) is included in an integrated model (KISSME). The model top is extended after that and the work which introduces stratosphere chemistry into a CHASER chemical process is done (the Heisei 16 - 17 fiscal year).
About stratosphere chemistry introduction, the halogenation study reaction (chlorine and bromine system) in the stratosphere and the heterogeneous reaction on polar stratosphere clouds (PSCs) are introduced on the basis of the stratosphere chemistry and the ozone hole model (Takigawa et al., 1999; Nagashima et al., 2002) developed in CCSR and NIES. In the framework of an integrated model, it assumes also expressing the interaction between a terrestrial ecosystem and atmospheric chemistry in consideration of the influence on the discharge process to the inside of the atmosphere of VOCs from vegetation and the self-possessed process (deposition) of the substance in the atmosphere by vegetation, and the vegetation according to the build-up of substances, such as nitric acid, further. In parallel to the above model construction work, the preceding paragraph story-experiment for joint future prediction of atmospheric chemistry and aerosol change, and warming is also conducted. For example, CHASER is used. IPCC SRES The future prediction experiment according to a scenario is started, and it is distribution of future ozone methane or sulfate aerosol. It is analyzing about what kind of cost performance the increase in emission (especially East Asia region) and warming of pollutant, such as NOx, CO, VOCs, SO2, have, respectively. (There is possibility, such as ozone destruction of a troposhere lower layer being promoted about the reaction by warming by the increase in steam, and generation of OH free radical from ozone increasing simultaneously, and affecting the upward tendency of methane) . The outline-schedule of the model development of this subgroup is shown in Fig. 24.
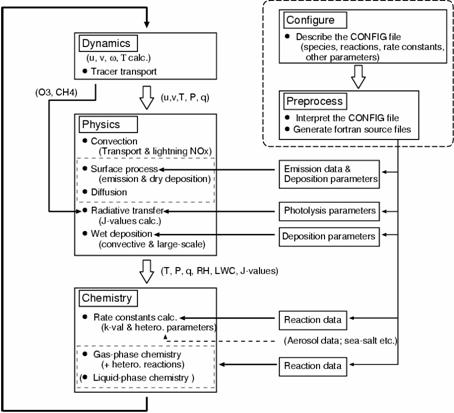
Fig. 23: Flow of CHASER model calculation. It is calculated on-line in CCSR/NIES AGCM about dynamics (transportation is included), a physical process, and each chemical process. A setup of chemical processes, such as a chemical reaction and addition of chemical species, is performed through a configuration file, and Fortran source code is generated automatically (cf., Sudo, 2003).
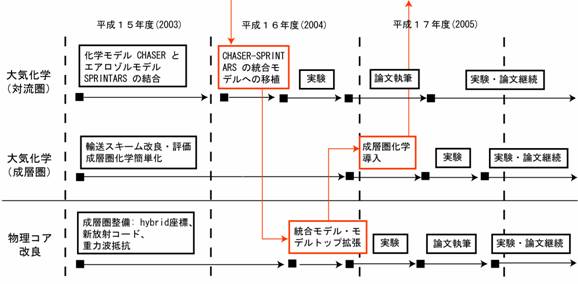
Fig. 24: Development work schedule of warming and atmospheric composition change interaction subgroup. A red line shows the flow of K2 integrated model development (KISSME).
d. The research program in the Heisei 17 fiscal yearIt is a chemistry model to an integrated model main part by the work of the former (the Heisei 16 fiscal year). CHASER and aerosol model Combination and introduction of SPRINTARS were performed. This fiscal year completes introduction of an aerosol thermodynamic equilibrium model about combination of calculation of troposhere chemistry and aerosol. Moreover, a halogenation study reaction and polar stratosphere cloud (PSCs) chemistry are added to CHASER in an integrated model, and it enables it to also perform the simulation of stratosphere ozone, after performing improvement in the formation of hybrid perpendicular coordinates of an integrated model, and a model top altitude under cooperation with a "climate physics core model improvement" group. e. Fruits of work in the Heisei 17 fiscal yeare.1. IPCC-SRES future prediction experiment (continuation) : emission / warming / stratosphere ozone change The last fiscal year is followed and it is IPCC/SRES. According to A2, A1, and B1 each scenario, the future prediction experiment of troposhere ozone, methane, and sulphate aerosol all ball distribution is conducted. The experiment was performed on the Earth Simulator (L system) using the chemical bond climate model CHASER. About what kind of influence change (recovery of an ozone layer) of the amount of stratosphere ozone upon which it will count in the future in addition to the influence of a future precursor gases emission change and a climate change has on a chemistry place (especially troposhere), this fiscal year was experimented in it and analyzed and evaluated in detail for every scenario. In this investigation, in order to separate each reaction of emission, a climate change, and stratosphere ozone change, three kinds of experiments, the experiment (Exp1) of only dishoarding (emission) diversification of ozone precursor gases, such as nitrogen oxide NOx and carbon monoxide CO, the experiment (Exp2) which also took the climate change into consideration in addition to emission diversification, and the experiment (Exp3) also in consideration of stratosphere ozone change (ozone layer rally) of further the future, were performed (Table 3). In these experiments, a future emission change and a climate change gave the sea surface temperature (SSTs) and sea ice (Sea-ice) distribution which were predicted by the CCSR/NIES air sea joint model about each scenario while making greenhouse effect gas concentration, such as CO2, both increase in a model about the climate change of Exp2 according to each scenario of A2 (increase size in emission), A1 (this Naka), and B1 (this smallness) of IPCC SRES. Table 3: Experiment scenario of future prediction by CHASER
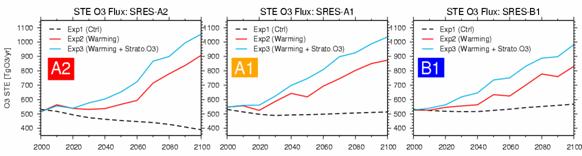
(Emission is taken into consideration about precursor gases of troposhere ozone, and CH4 and SO2) *2 Give with the function of all ball halogen total amounts (available chlorine conversion, EESCl) (IPCC-TAR). moreover, Exp3 simplifies and takes into consideration change (ozone layer rally) of the amount of stratosphere ozone which may happen in the future in addition to a setup of Exp2. (Means) It is as a function of the total amount of ball halogen on the basis of the ozone data of semiautomatic ground environment-I/II-TOMS about the east-and-west par ozone concentration place of the advanced area beyond the tropopause (in fact area of O3>500ppbv).It predicts beforehand (the means of D.Karoly and D.Sexton: Randel and Wu (1999)),This predicted value is made to ease the east-and-west par ozone place in CHASER (the scenario advocated by IPCC-TAR as time growth of the total amount of ball halogen was used). Fig. 25 is time development of the ozone inflow of the net from the stratosphere calculated in this experiment to the troposhere. Although it is stratosphere ozone inflow change reflecting the amount of ozone of the troposhere in the experiment (Exp1) of only emission change like the experiment conducted in the last fiscal year, when stratosphere circulation (Brewer-Dobson circulation) and troposhere circulation (Hadley circulation) were strengthened with the experiment (Exp2) also in consideration of a climate change in connection with warming and stratosphere -> troposhere transportation activated, it is 30-40% of increase in 100 years about every scenario. Especially in the experiment (Exp3) supposing future ozone layer (stratosphere ozone) recovery, if it is in 2020 and afterwards ‚³ et al., the increase in stratosphere ozone inflow is calculated, and in every scenario, 15-20% of increase is shown in 2100 compared with Exp2.
Fig. 25: Time development of the stratosphere/amount of ozone exchange between the troposhere (STE, net stratosphere -> troposhere ozone inflow) (TgO3/yr). A figure will show the result of Exp 1, 2, and 3 about SRES-A2, A1, and B1 each scenario even in 2000 to 2100, respectively.
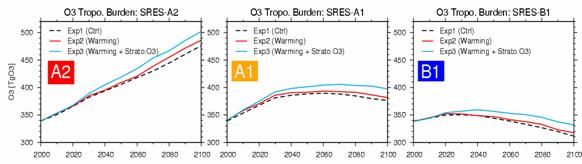
Fig. 26: Time development of ozone all ball total amount between the troposhere (TgO3) (2000 - 2100). The result of Exp 1, 2, and 3 is shown about SRES-A2, A1, and B1 each scenario, respectively. Table 4: Influence which each factor other than emission has on amount of troposhere ozone
* Although not only the increase in the amount of STE(s) but the effect which prolongs the photochemistry-life of the ozone in the troposhere through the ultraviolet light reduction to the troposhere has the influence on the amount of troposhere ozone by the increase in stratosphere ozone, in this experiment, it is not quantifying individually.
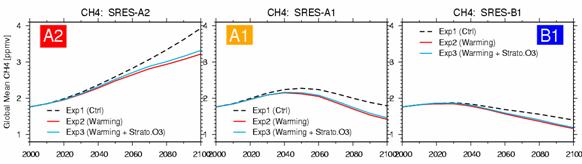
The difference in time development of each scenario and the ozone inflow from the above stratosphere in each experiment also influences time change of the amount of troposhere ozone not a little (Fig. 26). Especially the increase in the future stratosphere ozone of Exp3 (recovery of an ozone layer) also influenced all the ball total amounts of troposhere ozone greatly, and has caused about 5% of increase in the amount of troposhere ozone in o'clock in 2100 compared with Exp2 (the increase in stratosphere ozone needs cautions for it to be also effective in decreasing the incidence ultraviolet light to the troposhere and making the photochemistry-life of troposhere ozone increase). The influence factors other than the emission of ozone precursor gases affect the amount of troposhere ozone was summarized in Table 4. In time development of the troposhere ozone total amount of Fig. 26, there is almost no difference between the experiment Exp1 of only emission change, and the experiment Exp2 also in consideration of warming, and the influence of warming seems not to be large apparently. However, this is the result of canceling, when the effect of the opposite direction of the increase in up troposhere ozone by the increase in stratosphere ozone inflow by the troposhere lower layer ozone depletion by the increase in steam accompanying Table 4 (1) warming and (2) warming is calculation of all ball ozone total amounts. Fig. 27 shows the calculation result of time development of all ball average methane similarly. OH according to the increase in steam in the troposhere by warming experiment (Exp2) as obtained in the experiment by the last fiscal year -- since methane destruction becomes strong by radical concentration rise, compared with the standard experiment (Exp1), every scenario is low about 20%, and o'clock of methane concentration is calculated in Exp2 2100 years. On the other hand, the future increase in stratosphere ozone currently taken into consideration by Exp3 decreases the ultraviolet light to the troposhere,
O3 +hυ (ultraviolet light) → O (1D) +O2 ..... (1) O (1D) +H2O → OH+OH .................... (2)
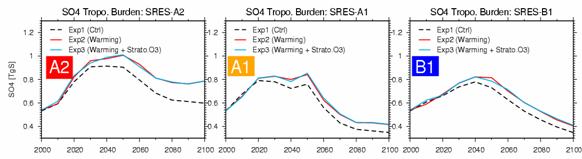
OH radical concentration produced at the reaction of the above-mentioned formula is controlled, and it can be forecasted that methane concentration becomes high relatively. However, the differences of the methane concentration of Exp2 and Exp3 were very few about every scenario in fact so that it might see to Fig. 27. This can be understood as the result with which the increase in the inflow to the troposhere of the stratosphere ozone accompanying the increase in stratosphere ozone of Exp3 as shown in Figs. 25 and 26, and the increase in the amount of troposhere ozone accompanying it strengthened the reaction of top (1) type in compensation. Furthermore, Fig. 28 shows time development of the troposhere total amount of sulphate aerosol. The influence of warming has appeared notably about every scenario in 2020 and afterwards, and this originates in strengthening of vapor phase oxidization (SO2+OH -> SO42-) of the sulfur dioxide (SO2) by the increase in OH radical accompanying warming, and strengthening of the liquid phase oxidization by the increase in an itinerant Buddhist. In the case of sulphate aerosol, since the great portion of generation process in the inside of the atmosphere is depended on the liquid phase reaction in an itinerant Buddhist, the influence of stratosphere ozone change is small, and by Exp3 and Exp2, a difference is hardly seen.
Fig. 27: Time development of total ball average methane concentration (ppmv) (2000 - 2100). The result of Exp 1, 2, and 3 is shown about SRES-A2, A1, and B1 each scenario, respectively.
Fig. 28: Time development of all troposhere ball total amounts (TgS) of sulphate aerosol (SO42-) (2000 - 2100). The result of Exp 1, 2, and 3 is shown about SRES-A2, A1, and B1 each scenario, respectively. The work which combines the chemistry model CHASER and the aerosol model SPRINTARS by work by the last fiscal year is completed. This fiscal year did the work (maintenance of data, such as emission of the addition of chemical species and a photochemical reaction, the correspondence to the new radiation code of the photodissociation constant calculation method and an improvement, and a halogenated compound etc.) which introduces a stratosphere chemical process into the CHASER model which was based on troposhere chemistry towards the unity coupling simulation (Fig. 29) of the aerosol and (stratosphere and troposhere) chemistry within the integrated model which is the target of this sub project. e.2.1. The addition of chemical species and a photochemical reactionSince it corresponded to the ozone chemistry in the stratosphere, chemical species were newly introduced for the halogen system compound as a center into CHASER. Table 5 shows the chemical species taken into consideration in CHASER at the time of the experiment included to stratosphere chemistry. About an Ox-NOx-HOx system, since there are much troposhere chemistry and across the board reaction, there is no big change. About NOx, the hydrogen atom H was newly taken into consideration about the nitrogen atom N and the HOx system. 25 kinds of chemical species were newly added for representation of a chlorine system (Cl) and bromine system (Br) compound or the ozone destructive process by suboxidation 2 nitrogen N2O. Thereby, by setup of only the troposhere, the number of chemical species which was 55 kinds increased with about 80 kinds, and increased from 38 kinds (troposhere chemistry center) to 64 kinds as the number of tracers.
Fig. 29: Fig. 29: Symbiosis 2nd : modeling of atmospheric chemistry and aerosol process in framework of earth system integrated model. It is put into the reaction of the radiation process on greenhouse effect gas, such as ozone methane, and the reaction of the cloud process on an aerosol kind by consideration including a detailed chemical reaction process and aerosol calculation. Table 5: Chemical-species list currently taken into consideration by CHASER (February, 2006 version)
* The chemical species (CFCs etc.) taken into consideration can be changed easily.
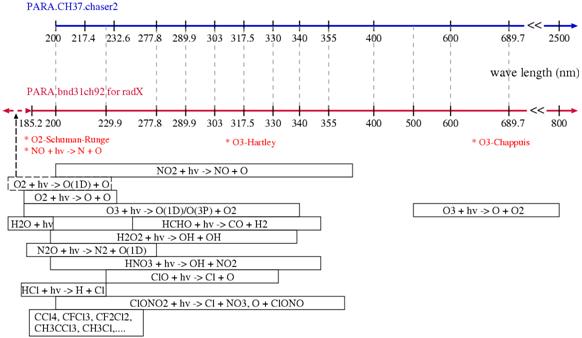
Table 6 shows the chemical reaction (only vapor phase reaction) of the stratosphere relation added by this work. The reaction following decomposition (mainly photodissociation) and it of CFCs, MCF, a carbon tetrachloride, and a chlorination and a methyl bromide is main, and serves as a 24 photodissociation reaction, a-39 kinetic reaction, and an addition of a total of about 63 as compared with version based on the troposhere (the number of chemical reactions is the whole and is 204). Although the chemical reaction of the stratosphere relation added this time is calculated simultaneously with troposhere chemistry by the chemistry scheme in CHASER (time quadrature), for the improvement in calculation speed, it also assumes calculating by separating the advanced domain of troposhere chemistry and stratosphere chemistry, respectively. e.2.2. Correction and improvement of the photodissociation constant calculation techniqueIn CHASER, calculation of the photodissociation constant in connection with a photodissociation reaction is calculated on-line in the radiation scheme in CCSR/NIES/FRCGC GCM. Therefore, it is necessary to prepare data, such as an absorption cross section required for calculation of a photodissociation constant, and a quantum yield, for every wavelength resolution currently taken into consideration by the radiation scheme of GCM. This fiscal year did the correspondence work to the new scheme of CHASER photodissociation constant calculation with renewal of the radiation scheme of CCSR/NIES/FRCGC GCM. The main improving points in a CCSR/NIES/FRCGC GCM new radiation scheme (mstrnX) : (1) Renewal to HITRAN2000 of a line absorption database from HITRAN92. (2) Change a continuum absorption program into MT_CKD_1 from the thing of LOWTRAN7. (3) Steep increase in a gas absorption band. (4) It is change of the optimization technique in the case of finding the integral point selection etc. By these updating, coincidence with Line by Line calculation becomes good compared with the case where the conventional radiation code is used, improvement in overall accuracy is attained (Sekiguchi 2004), and it contributes to the low-temperature bias dissolution in the lower stratosphere especially (refer to result report "climate physics core model improvement" in the H 15 fiscal year). At this new radiation scheme, it is possible to choose the wavelength resolution improved for atmospheric chemistry, and the maintenance of data and the improvement of the photodissociation constant calculation method corresponding to this wavelength resolution were performed by the updating work of this photodissociation constant calculation technique. The wavelength resolution of what recut the wavelength resolution of the old radiation code to CHASER (conventional version), and a new radiation code is compared with Fig. 30. The shortwave domain which it was to 200nm is taken to 185nm to the former, and the photodissociation in the Schuman-Runge belt of oxygen (O2) and the photodissociation process of N2O or CFCs can calculate now more correctly.
Fig. 30: The absorption wavelength domain of radiation code wavelength resolution and an important photodissociation reaction; old code (parameter file: PARA.CH37.chaser2) and new code ( PARA.bnd31ch92 for radX). Moreover, 800nm is adopted as a delimiter of the 690nm next, and it is easy to treat absorption with the Chappuis belt of ozone in the long wavelength side compared with a version conventionally whose following delimiter which is 690nm was 2500nm. In addition, the 400-600nm domain of a version is equally divided into two conventionally, and the accuracy of troposhere photochemistry calculation is raised. In calculation of a photodissociation constant, an absorption cross section and a quantum yield are given as follows to each substance about the above wavelength resolution (bottle). :
Although the module spectrum (F0) used the sunlight spectrum after letting the ozone column of 300 Dobson modules (DU) pass to each wavelength area uniformity in CHASER based on troposhere chemistry, since it needed to correspond also to stratosphere chemistry, it made the module spectrum variable for every wavelength area by this work. Moreover, although there is temperature dependency fundamentally about an absorption cross section or a quantum yield, about this, it calculates on-line in CHASER. Distribution of shortwave and infrared absorption gas, such as ozone calculated by the chemical process of CHASER, methane, and N2O, is passed to a radiation scheme on-line, and is reflected in radiation process. In addition, it is necessary to take into consideration the photodissociation reaction of the following methane by Lyman-alpha rays, steam, and an oxygen molecule. : CH4 +hυ → CH3 +H ........... (4)
H2O+hυ → H+OH ............... (5)
O2 +hυ → O (1D) +O ............ (6)
Since it cannot respond, about these, it is under examination by the present radiation scheme to give using parameterization (Brasseur and Solomn, 1984).
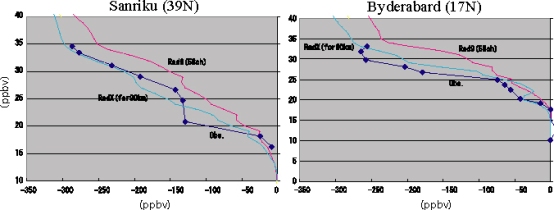
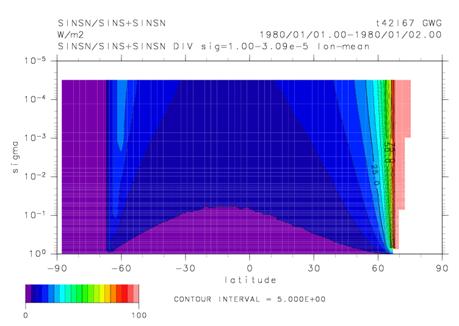
Fig. 31: Vertical distribution of N2O in the offing of Sanriku (left figure), and Hyderabad (right figure). A blue circle is the observation acquired by balloon observation. red line is a model calculation result in the old radiation scheme. blue line shows the model calculation result in a new radiation scheme, respectively. A unit is ppbv and is based on the concentration in surface of the earth. An improvement of the simulation of N2O is shown in Fig. 31 as an example at the time of using the wavelength resolution an above-mentioned new radiation scheme (mstrnX) and for CHASER. In the calculation using the conventional radiation scheme, since the photodissociation of N2O by radiation of a wavelength shorter than 200nm is disregarded, the observed value has been overestimated in the up stratosphere and a mesosphere, but such a tendency is canceled in the new scheme including the wavelength domain of 185 to 200 nm. Furthermore, in calculation of the photodissociation constant of a high latitude belt, the effect of an air surface of a sphere is especially important, and examination is required. This fiscal year introduced the surface-of-a-sphere effect to photodissociation speed based on Kurokawa et al. (2005) in order to reproduce the photochemical reaction in the circumference part of a polar vortex in detail. The rate of occupying to all the short-wave-radiation flux of the shortwave flux by the surface-of-a-sphere effect is shown in Fig. 32. It turns out that solar flux is increasing notably according to the surface-of-a-sphere effect from the stratosphere to a mesosphere especially in the portion of the polar night state of north north latitude 60 degrees. Since the solar flux in a polar-vortex circumference part may accelerate the ozone destruction by the halogenated compound generated by the heterogeneous reaction on the polar stratospheric cloud surface etc. within the polar vortex, it is due to evaluate further from now on about the influence which the surface-of-a-sphere effect has on distribution of a chemical substance.
Fig. 32: Latitude-advanced sectional view of rate (%) which influence of surface-of-a-sphere effect in all shortwave flux occupies. A contour interval is 5%.
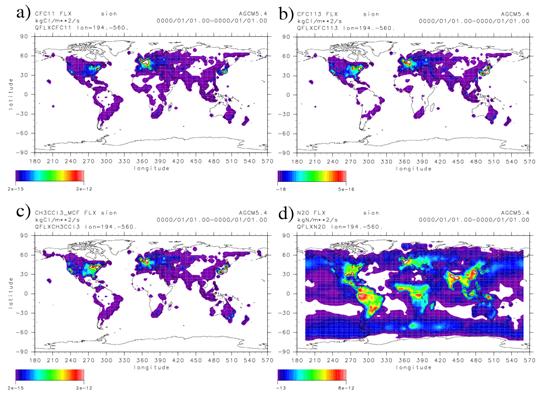
Fig. 33: The example of the emission data of the stratosphere related chemical species currently taken into consideration by CHASER : (a) CFC-11, (b) CFC-113, (c) methyl chloroform (MCF), and (d) N2O. chlorine system compounds (a, b, c) are chlorine atom conversion. (kgCl m-2 sec-1) . For the more realistic simulation of stratosphere ozone related chemistry, a halogen system compound and the emission data of N2O were fixed. Data processed it into the GCM (CHASER, T42) input using GEIA and an EDGAR inventory. The emission of industry and the traffic origin, or the forest fire and the agricultural origin is included in data. However, since it is also necessary to calculate by fixing each substance concentration in the troposhere (or surface of the earth) for convenience in case the long-term experiment of prediction etc. will be conducted in the future, it is also possible to experiment by inputting surface-of-the-earth fixed concentration. f. ConsiderationThis fiscal year performed a real trial and detailed evaluation first about the influence a future emission change, a climate change (warming), and stratosphere ozone change affect troposhere ozone and methane by the chemistry model CHASER. Although the same result as the experiment which the influence which emission change and warming do is conducting by the last fiscal year was obtained, in the experiment of this fiscal year, it was shown clearly that it is the factor which the amount change of stratosphere ozone (recovery of an ozone layer) cannot disregard further, either. However, in this experiment, stratosphere ozone change is diagnostically given by the simplified method, and the unity coupling of the stratosphere chemistry and troposhere chemistry in the inside of CHASER is required of the meaning of the interaction of the troposhere and stratosphere chemistry, and the interaction of atmospheric chemistry and climate. In response to such knowledge, this fiscal year did the work which introduces a stratosphere chemical process into the CHASER chemistry model which was based on troposhere chemistry further, and enables it to also perform the simulation of stratosphere ozone simultaneously. Although introduction of a stratosphere-related chemical species and a chemical reaction and improvement of the photodissociation constant calculation method were performed to the CHASER model in the work of this fiscal year, neither the heterogeneous reaction in the stratosphere nor introduction of an ozone hole chemistry scheme is completed. In the following fiscal year (H18 fiscal year), while completing introduction of the ozone hole scheme and heterogeneous reaction which are adopted by Akiyoshi et al (2004) etc. and performing unity coupling of the stratosphere and troposhere chemistry, it is due to adjust about the air surface-of-a-sphere effect in a gravity wave resistance scheme or radiation process. g. BibliographyAkiyoshi H., T. Sugata. S. Sugita, H. Nakajima, H. Hayashi, J. Kurokawa, M. Takahashi, Lower-N2O air masses after the breakdown of the arctic polar vortex in 1997 simulated by the CCSR/NIES nudging CTM, J. Meteorol. Soc. Jpn, 80, 451-463, 2002. Akiyoshi H., T. Sugita, H. Kanzawa, N. Kawamoto (2004), Ozone perturbations in the Arctic summer lower stratosphere as a reflection of NO X chemistry and planetary scale wave activity, J. Geophys. Res., 109, D03304, doi:10.1029/2003JD003632. Brasseur, G., and S. Solomon, Aeronomy of the Middle Atmosphere, 1984. Kurokawa J., H. Akiyoshi, T. Nagashima, H. Masunaga, T. Nakajima, M. Takahashi, H. Nakane (2005), Effects of atmospheric sphericity on stratospheric chemistry and dynamics over Antarctica, J. Geophys. Res., 110, D21305, doi:10.1029/2005JD005798. Nagashima, T., M. Takahashi, M. Takigawa, and H. Akiyoshi, Future development of the ozone layer calculated by a general circulation model with fully interactive chemistry, Geophys. Res. Lett., 29, 10.1029/2001GL014926, 2002. Nagashima, T., M. Takahashi, H. Akiyoshi, and M. Takigawa, The effects of non-orographic GWD scheme and radiation from large SZA on the Antarctic ozone hole, Process-oriented validation of coupled chemistry-climate models, 2003. Randel, W. J., and F. Wu, A stratospheric ozone trends data set for global modelling studies, Geophys. Res. Lett., 26 no.20, 3089-3092, 1999. Sudo, K. and M. Takahashi, Simulation of tropospheric ozone changes during 1997-1998 El Nino: Meteorological impcat on tropospheric photochemistry, Geophys. Res. Letters., 28, 4091-4094, 2001. Sudo, K., M. Takahashi, J. Kurokawa, and H. Akimoto, CHASER: A global chemical model of the troposphere 1. Model description, J. Geophys. Res., 107, 10.1029/2001JD001113, 2002a. Sudo, K., M. Takahashi, and H. Akimoto, CHASER: A global chemical model of the troposphere 2. Model results and evaluation, J. Geophys. Res., 107, 10.1029/2001/JD001114, 2002b. Sudo, K., Changing process of global tropospheric ozone distribution and related chemistry: a study with a coupled chemistry GCM, Doctoral Dissertation, Dept. of Earth and Planetary Science, Graduate School of Science, Univ. of Tokyo, 187pp, 2003. Sudo, K., M.Takahashi, and H.Akimoto, "Future changes in stratosphere-troposphere exchange and their impacts on future tropospheric ozone simulations", Geophysical Research Letters., 30, 24, 2256, doi:10.1029/2003GL018526, 2003. Takemura, T., T. Nakajima, O. Dubovik, B.N. Holben, and S. Kinne, Single-Scattering albedo and radiative forcing of various aerosol species with a global three-dimensional model, Journal of Climate, 15, 4, 333-352, 2002. Takigawa, M., M. Takahashi, and H. Akiyoshi, Simulation of ozone and other chemical species using a center for climate system research/national institute for environmental studies atmospheric GCM with coupled stratospheric chemistry, J. Geophys. Res., 104, 14,003-14,018, 1999. Junichi Kurokawa, Eiji Akiyoshi, Tatsuya Nagashima, Hideaki Nakane, Hirohiko Masunaga, Teruyuki Nakajima, Masaaki Takahashi : CCSR/NIES Induction of the air spherical-surface cost performance to a stratosphere nudging chemistry transportation model and a photochemistry joint model. the collection of the Meteorological Society of Japan spring rally lecture drafts in the 2002 fiscal year, and p -- 346 and 2002. Sekiguchi Miho, the University of Tokyo doctoral dissertation, and 2004. h. The announcement of a result<Paper announcement> Dentener F., D.Stevenson, K.Ellingsen, T.van Noije, M.Schultz1, M.Amann, C.Atherton, N.Bell, D.Bergmann, I.Bey, L.Bouwman, T.Butler, J.Cofala, B.Collins, J.Drevet, R.Doherty, B.Eickhout, H.Eskes, A.Fiore, M.Gauss, D.Hauglustaine, L.Horowitz, I.Isaksen, B.Josse, M.Lawrence, M.Krol, J.F.Lamarque, V.Montanaro, J.F.Muller, V.H.Peuch, G.Pitari, J.Pyle, S.Rast, J.Rodriguez, M.Sanderson, N.H.Savage, D.Shindell, S.Strahan, S.Szopa, K.Sudo, R.Van Dingenen, O.Wild, G.Zeng, The global atmospheric environment for the next generation, Environmental Science & Technology, in press, 2005. Gauss, M. , Myhre, G., Isaksen, I. S. A., Grewe, V., Pitari, G., Wild, O., Collins, W. J., Dentener, F. J., Ellingsen, K., Gohar, L. K., Hauglustaine, D. A., Iachetti, D., Lamarque, J. -F., Mancini, E., Mickley, L. J., Prather, M. J., Pyle, J. A., Sanderson, M. G., Shine, K. P., Stevenson, D. S., Sudo, K., Szopa, S. and Zeng, G., Radiative forcing since preindustrial times due to ozone change in the troposphere and the lower stratosphere, Atmospheric Chemistry and Physics, Vol. 6, pp 575-599, 24-2-2006. Irie, H., K. Sudo, H. Akimoto, A, Richter, J.P. Burrows, T. Wagner, M. Wenig, S. Beirle, Y. Kondo, V.P. Sinyakov, and F. Goutail, Evaluation of long-term tropospheric NO2 data obtained by GOME over East Asia in 1996-2002, Geophys. Res. Letters., 32, L11810 doi:10.1029/2005GL022770, 2005. Kawamiya, M., C. Yoshikawa, T. Kato, H. Sato, K. Sudo, S. Watanabe, and T. Matsuno, Development of an Integrated Earth System Model on the Earth simulator, J. Earth Sim., 4, 2005. Stevenson D.S., F.J. Dentener, M.G. Schultz, K. Ellingsen, T.P.C. van Noije, O. Wild, G. Zeng, M. Amann, C.S. Atherton, N. Bell, D.J. Bergmann, I. Bey, T. Butler, J. Cofala, W.J. Collins, R.G. Derwent, R.M. Doherty, J. Drevet, H.J. Eskes, A.M. Fiore, M. Gauss, D.A. Hauglustaine, L.W. Horowitz, I.S.A. Isaksen, M.C. Krol, J.-F. Lamarque, M.G. Lawrence, V. Montanaro, J.-F. Muller, G. Pitari, M.J. Prather, J.A. Pyle, S. Rast, J.M. Rodriguez, M.G. Sanderson, N.H. Savage, D.T. Shindell, S.E. Strahan, K. Sudo, and S. Szopa, Multi-model ensemble simulations of present-day and near-future tropospheric ozone, J. Geophys. Res., in press, 2005. M.G. Lawrence, O. Hov, M. Beekmann, J. Brandt, H. Elbern, H. Eskes, H. Feichter, and M. Takigawa, The Chemical Weather, Environ., Chem., 2, 6-8, doi:10,1071/EN05014, 2005. Takigawa, M, K. Sudo, H. Akimoto, K. Kita, N. Takegawa, Y. Kondo, and M. Takahashi: Estimation of the contribution of intercontinental transport during PEACE campaign by using a global model, J. Geophys. Res., 110, D21313, doi:10.1029/2005JD006226, 2005. <Oral announcement> Sudo, K., Akimoto H., and Takahashi M., Source attribution of global tropospheric O3 and CO: where do they come from ?, International Association of Meteorology and Atmospheric Sciences, Beijing, China, 1-11 August, 2005. Sudo, K., Akimoto H., and Takahashi M., Past/Future Climate change impacts on atmospheric chemistry in a chemistry coupled climate model, 1st ACCENT Symposium, Urbino, Italy, 12-16th September, 2005. Sudo, K., Atmospheric chemistry and aerosols modeling in the FRCGC Earth System model, 1st German-Japan Workshop on Numerical Climate Modeling, Kashiwa, Chiba, Japan, 31st Oct - 1st Nov , 2005. Sudo, K., Takigawa M., Nagashima T., and Takahashi M., Chemistry-Aerosol modeling in the FRCGC Earth System Model, 1st UJCC International Workshop on Current Problems in Earth System Modelling, Yokohama, Japan, 24-25th Nov., 2005. Kengo Sudo, Hajime Akimoto: "origin of the all ball distribution and income and expenditure of ozone and CO, and all ball scale long hauls", the 16th atmospheric-chemistry symposium, Toyokawa, 11-January 13, 2006. Masayuki Takigawa, "a substance transportation verification experiment in the CCSR/NIES general circulation model using an airmass date spectrum", ozone nexus study group, Kobe, November, 2005. Next page (2.2 A warming-cloud, aerosol, and radiation feedback precision evaluation) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

![Fig. 29: the [ symbiosis ] -- the modeling of the atmospheric chemistry and aerosol process in the framework of 2 and an earth system integrated model.](./figure/2005-29.jpg)