Press Releases
August 5, 2013
JAMSTEC
Agilent Technologies
IFREMER
A high-accuracy analysis for 236U using ICP mass spectrometry
—Contributing to detailed understanding of paleo climate change through rapid U-Th dating of marine calcium carbonates—
1. Overview
Masaharu Tanimizu, a sub-leader at the Kochi Institute for Core Sample Research Geochemical Research Group of the Japan Agency for Marine-Earth Science and Technology (JAMSTEC) (President: Asahiko Taira), has established a rapid high-accuracy analytical method for trace 236U (*1) using a desktop-type ICP mass spectrometer for dating of coral and other marine calcium carbonates using the 234U-230Th radiometric dating method (*2). The work was carried out in cooperation with Agilent Technologies Inc. and the French Research Institute for Exploitation of the Sea (IFREMER). This method improves ion beam convergence, which was an issue associated with the conventional ICP mass spectrometry (*3). Markedly more rapid and easier high-accuracy analysis of 236U were achieved compared with other mass spectrometry (*4) by selectively removing interference molecule ions (U hydride ions, etc.). Furthermore, by applying this method to 234U-230Th dating, the research team expects that rapid, high precision dating will be possible.
These findings were published in the Journal of Analytical Atomic Spectrometry, a journal dedicated to the spectrometry field published by the Royal Society of Chemistry.
Title: Determination of ultra-low 236U/238U isotope ratios by tandem quadrupole ICP-MS/MS Authors: Masaharu Tanimizu1, Naoki Sugiyama2, Emmanuel PONZEVERA 3, Germain BAYON 3
Affiliations: 1. Kochi Institute for Core Sample Research, Japan Agency for Marine-Earth Science and Technology, 2. Agilent Technologies Japan, Ltd., 3. IFREMER, Unité de Recherche Géosciences Marines
URL: http://dx.doi.org/10.1039/c3ja50145k
*1 236U: In general not occurring naturally, a manmade nuclide formed by the nuclear reaction of 235U with a neutron. Half-life is 23.42 million years. In the reaction of 235U with thermal neutrons, more than 10% do not undergo nuclear fission and are converted to 236U.
*2 234U-230Th dating method: A dating method that utilizes the radioactive decay of 234U (half-life: 246,000 years), found in trace quantities in marine calcium carbonates such as coral, into 230Th (half-life: 75,400 years). Here, when the half-life of the nuclide prior to decay (parent nuclide) is significantly longer than that of the nuclide after decay (daughter nuclide), a so-called secular equilibrium state arises in which the quantity of radiation emitted by each nuclide is equivalent. The half-life of the 234U parent nuclide is much longer than that of the 230Th daughter nuclide and on this account, a secular equilibrium state is approached as the 234U radioactive decays to 230Th. Here, if a change arises in the abundance ratios of U and Th including both of these nuclides through geological phenomena, the secular equilibrium state is at first broken but after some time, it gradually approaches to a new secular equilibrium state. In this method, dating is carried out by measuring differences in the degrees of the decay rates between the two nuclides in the in the approaching process.
*3 ICP mass spectrometry: A mass spectrometry that uses inductively coupled plasma (ICP) as the ion source. An aerosol of samples is introduced into a high temperature argon plasma (approximately 8000°C) for ionization. At this temperature high ionization efficiencies can be achieved regardless of the first ionization potential of elements. On the other hand, the initial kinetic energies of the ions obtained are high and for this reason, ion beam convergence is inferior to the thermal ionization mass spectrometry. Furthermore, various polyatomic interference molecule ions other than the target elements that are derived from argon as well as nitrogen, oxygen and water in the air are formed, and they can easily generate background noise in the mass spectrum.
*4 Mass spectrometry: A method whereby a target element is ionized using various ion sources, accelerated through an electric field, and then separated through magnetic or electric fields, and thereby detected according to individual isotopic mass number. The mass spectrum is a plot of the number of ions detected for each mass number for the target element. The interior of the instrument is kept under a high vacuum condition not to lose ions produced at the ion source due to collisions with residual gases.
Figure 1: Exterior appearance of Agilent 8800 mass spectrometer (reproduced from Agilent Technologies website)
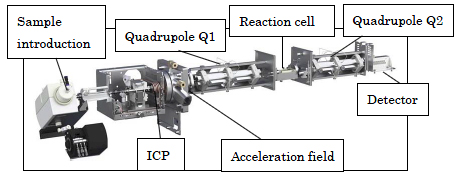
Figure 2: Enlarged view of Agilent 8800 mass spectrometer and explanation of each component (reproduced from the Agilent Technologies website)
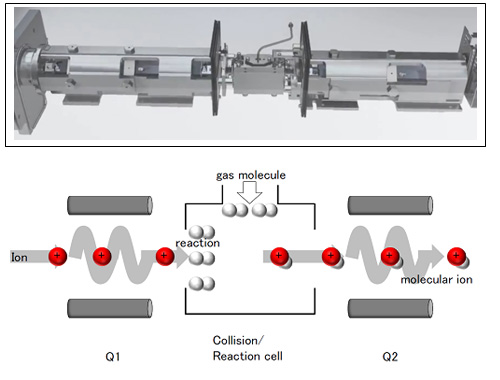
Figure 3: Enlarged view of Agilent 8800 mass spectrometer section (first quadrupole electric field Q1-ion reaction cell- second quadrupole electric field Q2) (reproduced from the Agilent Technologies website) and schematic diagram.
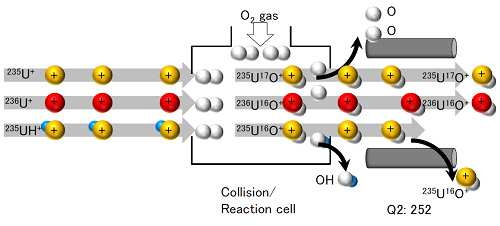
Figure 4: Outline of mechanism for high-accuracy detection of 236U ions. (a) shows a conventional ICP mass spectrometer while (b) shows the Agilent 8800 spectrometer used in the current research. Q1 and Q2 indicate quadrupole electric fields; they allow only ions of specific mass to pass through. When a conventional ICP analytical apparatus ((a) in the figure) does not use reaction gas, no fractionation is made between ions with the same mass number such as 236U+ and 235UH+ and they end up being detected together. In addition, while 235UH+ can be removed by reaction with oxygen gas, no distinction is possible between the 235U17O+ newly produced in the reaction cell and 236U16O+. In contrast, the Agilent 8800 spectrometer ((b) in the figure) has a quadrupole electric field before the reaction cells and because 235U+ is thus excluded from the reaction cell by Q1, 235U17O+ is not formed. Furthermore, because 235UH+ ions are converted to 235U16O+ ions of different mass through reaction with oxygen gas, it is possible to detect only the target 236U16O+ ions with high accuracy. (a) Conventional ICP mass spectrometer
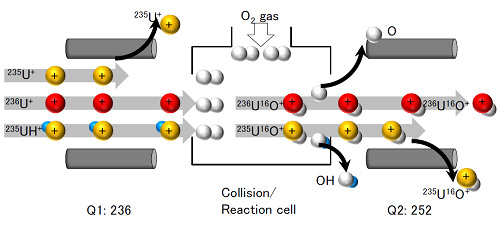
(b) Agilent 8800 mass spectrometer
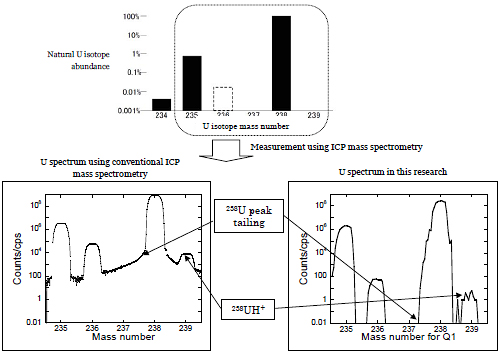
Figure 5: Natural U isotope abundance and uranium isotope mass spectrum obtained from an Agilent 8800 mass spectrometer, plus comparison with conventional spectrum. The U spectrum in this research exhibits a markedly clearer peak shape, and uranium hydride ions (238UH+) are remarkably suppressed. (U16O+ spectrum actually obtained from the Agilent 8800 mass spectrometer was converted to a U+ spectrum for an easy comparison). Many of the reagents containing uranium that are currently commercially available partly contain uranium reprocessed from burned nuclear fuels and in some cases, the isotopic composition is different from that which occurs naturally.
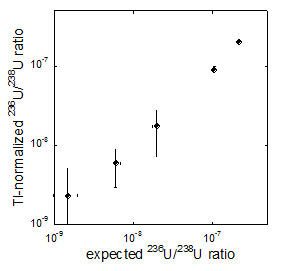
Figure 6: Comparison of expected (horizontal axis) and obtained (vertical axis) 236U/238U ratios (from 10-9 to 10-7). For reagents containing uranium where the 236U/238U ratio are already known, their uranium isotope ratios were measured using the Agilent 8800 mass spectrometer and the correlation was evaluated. The recommended values (horizontal axis) and values actually obtained (vertical axis) for the reagent 236U/238U ratios exhibit a 1:1 relationship (points of the same value are plotted on a straight line with a slope of one), and the correlation values are found to be in good agreement. From this figure, one can say that a 236U/238U ratio of 10-9, in other words, 1 236U atom per 1 billion 238U atoms, can be detected.
Contacts:
Japan Agency for Marine-Earth Science and Technology (JAMSTEC)
- (For the study)
-
Masaharu Tanimizu, Sub Leader
Geochemical Research Group, Kochi Institute for Core Sample Research (KOCHI) - (For publication)
-
Kazushige Kikuchi, Director
Planning Department Press Office
E-mail: press@jamstec.go.jp
