Press Releases
September 11, 2014
JAMSTEC
Greenhouse Gas*1 Emission Pattern May Need To Be Revised
〜Comprehensive Evaluation of Hydroxyl Radical (OH)*2 Distribution 〜
1.Overview
A joint research team led by Dr. Prabir K Patra, Senior Scientist, Department of Environmental Geochemical Cycle Research, Japan Agency for Marine-Earth Science and Technology (JAMSTEC: Asahiko Taira, President) determined the Northern Hemispheric (NH) to Southern Hemispheric (SH) ratio of the hydroxyl radical (OH) concentration by using JAMSTEC's Atmospheric Chemistry Transport Model (ACTM)*3, highly-accurate observations from surface networks and aircraft campaigns. To estimate the OH concentration ratio, various evaluation methods have been developed since the 1990s; however, this is the first attempt to apply such a multidimensional approach, which accounts for uncertainties in both the model transport and emissions of methyl chloroform (CH3CCl3).
It has been assumed that OH concentration in the NH, where more chemical substances are emitted, was higher than that in the SH based on simulations using chemical transport models. This research result revealed that the average OH concentrations in the NH and the SH is almost the same. It suggests that the hemispheric (and thus sectorial) emissions of greenhouse gases and air pollutant species in the existing chemistry-climate models need be reviewed.
A better understanding of the NH/SH OH concentration ratio in the atmosphere will contribute to accurate simulation of meridional concentration gradients and unbiased estimation of emission distributions of chemical substances. This is because, as the primary atmospheric oxidant, the OH plays a key role in the removal or production of major air pollutants, greenhouse gases (e. g., methane*4), and many ozone depleting substances.
This study has been carried out as part of Grant-in-Aid for Scientific Research (A) (Research Number: 22241008), the Japan Society for the Promotion of Science and Arctic Climate Change Research, Green Network of Excellence (GRENE) Project (led by National Institute of Polar Research) by Ministry of Education, Culture, Sports, Science and Technology. It was published in Nature on the 11th September 2014 (0200 JST).
Title: Observational evidence for interhemispheric hydroxyl parity
Authors:P.K. Patra1,2, M.C. Krol3, S.A. Montszka4, T. Arnold5, E.L. Atlas6, B.R. Lintner7, B.B. Stephens8, B. Xiang9, J.W. Elkins4, P.J. Fraser10, A. Ghosh11,1, E.J. Hintsa4,12, D.F. Hurst4,12, K. Ishijima1, P.B. Krummel10, B.R. Miller4,12, K. Miyazaki1, F.L. Moore4,12, J. Muhle5, S. O'Doherty13, R.G. Prinn14, L.P. Steele10, M. Takigawa1, H.J. Wang15, R.F. Weiss5, S.C. Wofsy9, D. Young13
1. Department of Environmental Geochemical Cycle Research, JAMSTEC
2. CAOS, Graduate School of Studies, Tohoku University
3. Wageningen University (The Netherlands)
4. National Oceanic and Atmospheric Administration (NOAA), (USA)
5. Scripps Institution of Oceanography, University of California (USA)
6. University of Miami (USA)
7. Rutgers, The State University of New Jersey (USA)
8. National Center for Atmospheric Research (NCAR), (USA)
9. Harvard University (USA)
10. Commonwealth Scientific and Industrial Research Organisation (CSIRO) (Australia)
11. National Institute of Polar Research
12. University of Colorado (USA)
13. University of Bristol (UK)
14. Massachusetts Institute of Technology (USA)
15. Georgia Institute of Technology (USA)
*1 Greenhouse gas: A gas in the atmosphere that absorbs infrared radiation emitted from the Earth's surface. The absorbed radiations are scattered back to the Earth's surface, traping heat in the atmosphere. The major greenhouse gases in the Earth's atmosphere are water vapour (H2O), carbon dioxide (CO2), methane (CH4), nitrous oxide (N2O), halocarbons and ozone (O3).
*2 Hydroxyl radical (OH): A group of reactive oxidant species. It is produced by the reaction of excited atomic oxygen (O1D) with water vapor in the air. Since many chemical reactions in the troposphere are initiated by OH reaction, OH concentration form an important factor for determining lifetime of the substances once emitted to the atmosphere. OH concentration in air is very low, but plays major role in chemistry of the troposphere since OH is recycled in a series of chemical reactions. OH is produced during ozone chemistry in the presence of nitrogen oxide and methane emissions from human activities. On the other hand, carbon monoxides and hydrocarbons emitted by human activities generally contribute to removal of OH.
*3 Atmospheric Chemistry Transport Model (ACTM): The ACTM is based on the CCSR/NIES/FRCGC atmospheric general circulation model (AGCM)-based chemistry-transport model developed in JAMSTEC. The ACTM is validated extensively for interhemispheric transport using simulation of sulfur hexafluoride (SF6), which is extremely stable in the troposphere and stratosphere. The model is run on the high performance computing facility in JAMSTEC for simulating temporal and spatial changes of various greenhouse gases and ozone depleting substances in the atmosphere. The model is being utilized for estimating regional sources and sinks of many of these atmospheric minor species for supporting international assessment activities.
*4 Methane (CH4):It is the most abundant hydrocarbon in the Earth's atmospheric. Methane is emitted mainly from swamps, bogs and rice paddies, enteric fermentation, gas and oil industry, waste management, biomass burning, and coal mining. It is the second most abundant anthropogenically produced greenhouse gas after carbon dioxide, producing large impacts on global warming. The lifetime of methane in the atmosphere is about 10 years. Like methyl chloroform, it is removed mainly by reaction with the hydroxyl radical. Since CH4 also participates actively in air pollution chemistry, reduction of CH4 emissions offers potential co-benefits for improving Earth's future environment.
Figure 1: Hydroxyl radical in the atmosphere: OH radicals are generated by a series of chemical reactions in the atmosphere, and have significant impacts on almost all chemical substances in the atmosphere.
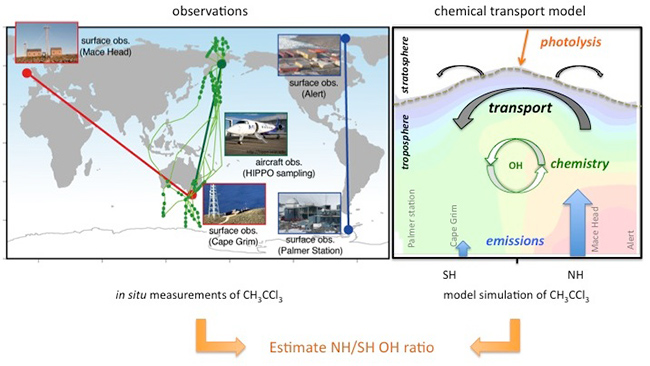
Figure 2: Methods used for estimation of NH/SH OH concentration ratio in this study. Measurements from the two surface observation networks (left panel; red: AGAGE, Advanced Global Atmospheric Gases Experiment, http://agage.eas.gatech.edu; blue: NOAA, National Oceanic and Atmospheric Administration, http://www.esrl.noaa.gov/gmd) and 5 aircraft campaigns (green: HIPPO, HIAPER Pole-to-Pole Observations, http://hippo.ucar.edu), and the JAMSTEC's ACTM (right panel) simulations are depicted schematically.
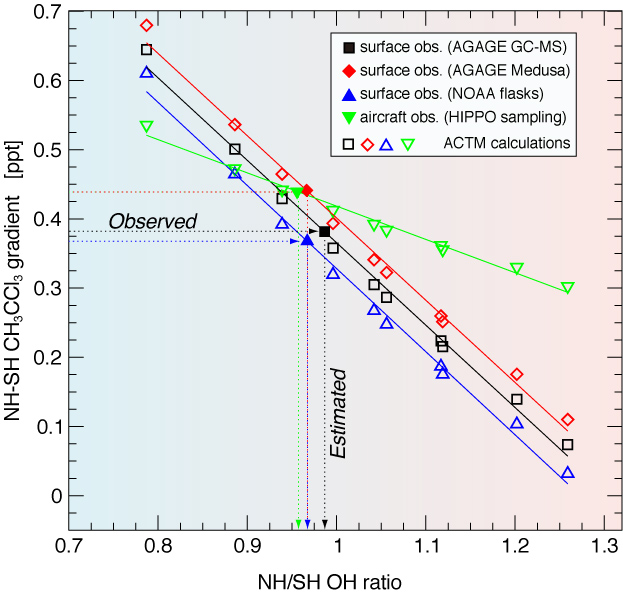
Figure 3: Estimation of the NH/SH OH concentration ratio from CH3CCl3 interhemispheric gradients. NH–SH CH3CCl3 concentration differences for different measurement data sets (see legends) and those based on the ACTM sensitivity simulations for various NH/SH OH concentration ratios during the period 2004–2011. The case of 'Control' global total emissions and global mean OH concentrations maintained for all ACTM simulations. The NH/SH OH concentration ratios are calculated from the fitted model lines corresponding to each of the observations.
Contacts:
- (For this study)
- Prabir K. Patra, Senior Scientist
Department of Environmental Geochemical Cycle Research - (For press release)
- Kazushige Kikuchi, Manager,
Press Division, Public Relations Department