Press Releases
JAMSTEC
University of the Ryukyus
Tokyo University of Marine Science and
Technology
Foraminifera Pumps Proton for Shell Formation
- To buffer seawater against pH changes due to ocean acidification -
Overview
A research team led by Dr. Takashi Toyofuku at Department of Marine Biodiversity Research & Dr. Miki Yamamoto-Matsuo at Department of Mathematical Science and Advanced Technology, the Japan Agency for Marine-Earth Science and Technology (JAMSTEC: Asahiko Taira, President) have found that foraminiferal calcification is driven by rapid transformation of bicarbonate into carbonate inside the cytoplasm with active outward proton pumping. Culturing experiments mimicking ocean acidification*i have also resulted in similar responses of calcification. The work was carried out in collaboration with NIOZ Royal Netherlands Institute for Sea Research and Utrecht University, University of the Ryukyus and Tokyo University of Marine Science and Technology.
Foraminifera, unicellular marine organisms, typically produce a shell, which is commonly made of calcium carbonate in seawater, though the mechanism has not been fully revealed. By visualizing extracellular pH around benthic perforate foraminifera, the scientists demonstrated that the external pH rapidly decreases during chamber formation. It means that foraminifera precipitate calcium carbonate by maintaining pH in the site of calcification with proton pumping. In addition, when proton pumping is artificially inhibited with chemical agent in the experiments, the chamber formation becomes inactive. These results indicate that proton pumping stimulate chamber formation process in foraminifera.
Ocean acidification is widely reported to reduce the ability of calcifying marine organisms to produce their shells and skeletons due to the reduction in carbonate saturation state as atmospheric CO2 levels increase. These findings will help further examine impacts of ongoing ocean acidification on other marine organisms like corals and shellfishes.
This work was supported by JSPS KAKENHI Grant Numbers 22684027459 and 25247085. The above results were published the online Nature Communications on January 27, 2017 (JST).
Title: Proton pumping accompanies calcification in foraminifera
Authors: Takashi Toyofuku1, Miki Y. Matsuo2, Lennart Jan de Nooijer3, Yukiko
Nagai1, Sachiko Kawada1, Kazuhiko Fujita4, Gert-Jan Reichart3,5, Hidetaka
Nomaki6, Masashi Tsuchiya1, Hide Sakaguchi2, Hiroshi Kitazato7
Affiliations: 1Department of Marine Biodiversity Research, JAMSTEC 2Department of Mathematical Science and Advanced Technology, JAMSTEC 3 NIOZ Royal Netherlands Institute for Sea Research and Utrecht University 4Department of Physics and Earth Sciences, Faculty of Science and Tropical Biosphere Research Center, University of the Ryukyus 5Department of Earth Sciences – Geochemistry, Faculty of Geosciences, Utrecht University 6Department of Biogeochemistry, JAMSTEC 7Tokyo University of Marine Science and Technology
*i Ocean acidification: Human activity produces 20Gt of CO2 per year, 30% of which is considered to be dissolved in seawater. According to reports by Intergovernmental Panel on Climate Change(IPCC), the pH in seawater is expected to decrease to 7.6-7.9 from the current 8.1 by 2100, indicating the progress of ocean acidification.
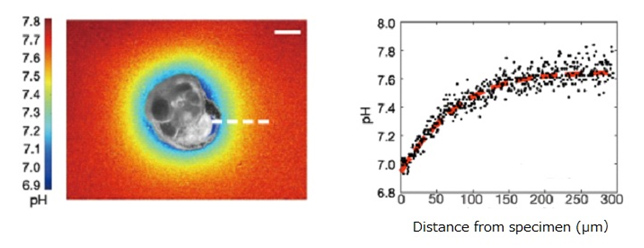
Figure 1. (Left) An incubated specimen shows the two-dimensional variability in pH around it when building a new chamber. pH is quantified based on digital image analysis. The scale bar indicates 50μm.
(Right) Translated and spatially-integrated change in pH versus distance from the foraminifer along the white dotted line shown in the left image. The red dotted line indicates a regression curve.
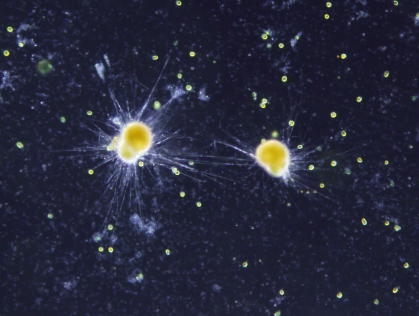
Figure 2. Foraminifera are single-celled marine organisms with shells.
The shells composed of secreted calcium carbonate are preserved as fossils.
Serving as index and facies fossils, they often provide environmental information in the past.
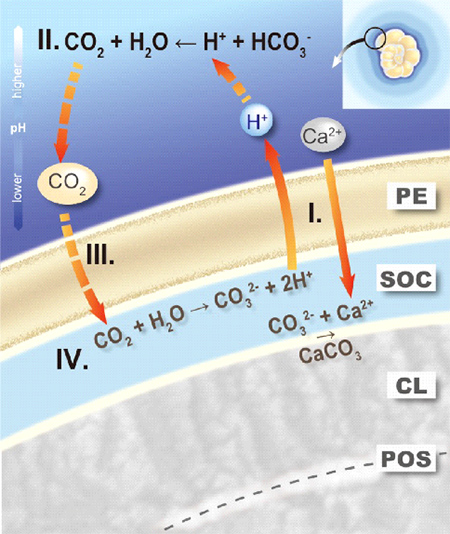
Figure 3. Proton pumping-based model of foraminiferal calcification.
During calcification of a new calcitic layer (CL) on a primary organic sheet (POS), the protective envelope (PE) separates the growing calcite surface from the surrounding seawater. The chemical composition at the site of calcification (SOC), created by the PE, is characterized by active, outward proton pumping (I). The reduced pH in the foraminiferal microenvironment shifts the inorganic carbon speciation (II), thereby increasing pCO2 directly outside the PE. The large gradient in pCO2 across the PE results in diffusion of CO2 into the SOC (III). Once inside, the CO2 reacts to form CO32- due to the high pH (IV) sustaining CaCO3 precipitation by reacting with the Ca2+.
Foraminifera Pumps Proton for Shell Formation
- To buffer seawater against pH changes due to ocean acidification -
Contacts:
- (For this study)
- JAMSTEC
- Takashi Toyofuku, Senior Scientist, Department of Marine Biodiversity Research
- University of the Ryukyus
- Kazuhiko Fujita, Professor, Department of Physics, Faculty of Science
- Tokyo University of Marine Science and Technology
- Hiroshi Kitazato, Specially Appointed Professor
- (For press release)
- JAMSTEC
- Tsuyoshi Noguchi, Manager, Press Division, Public Relations Department
- University of the Ryukyus
- Hanae Yamashiro, General Affairs Unit, Faculty of Science
- Tokyo University of Marine Science and Technology
- Public Relations Division, General Affairs Department
